Sorting Nexins in Protein Homeostasis
- PMID: 33374212
- PMCID: PMC7823608
- DOI: 10.3390/cells10010017
Sorting Nexins in Protein Homeostasis
Abstract
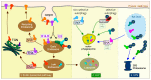
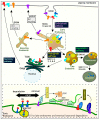
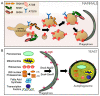
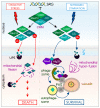
Protein homeostasis is maintained by removing misfolded, damaged, or excess proteins and damaged organelles from the cell by three major pathways; the ubiquitin-proteasome system, the autophagy-lysosomal pathway, and the endo-lysosomal pathway. The requirement for ubiquitin provides a link between all three pathways. Sorting nexins are a highly conserved and diverse family of membrane-associated proteins that not only traffic proteins throughout the cells but also provide a second common thread between protein homeostasis pathways. In this review, we will discuss the connections between sorting nexins, ubiquitin, and the interconnected roles they play in maintaining protein quality control mechanisms. Underlying their importance, genetic defects in sorting nexins are linked with a variety of human diseases including neurodegenerative, cardiovascular diseases, viral infections, and cancer. This serves to emphasize the critical roles sorting nexins play in many aspects of cellular function.
Keywords: autophagy; endosome; lysosome; proteasome; retromer; sorting nexins; ubiquitin.
Conflict of interest statement
The authors declare no competing or financial interests.
Figures

References
-
- Khandia R., Dadar M., Munjal A., Dhama K., Karthik K., Tiwari R., Yatoo M.I., Iqbal H.M.N., Singh K.P., Joshi S.K., et al. A Comprehensive Review of Autophagy and Its Various Roles in Infectious, Non-Infectious, and Lifestyle Diseases: Current Knowledge and Prospects for Disease Prevention, Novel Drug Design, and Therapy. Cells. 2019;8:674. doi: 10.3390/cells8070674. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

