Bone Regeneration Capacity of Newly Developed Uncalcined/Unsintered Hydroxyapatite and Poly-l-lactide-co-glycolide Sheet in Maxillofacial Surgery: An In Vivo Study
- PMID: 33374294
- PMCID: PMC7824502
- DOI: 10.3390/nano11010022
Bone Regeneration Capacity of Newly Developed Uncalcined/Unsintered Hydroxyapatite and Poly-l-lactide-co-glycolide Sheet in Maxillofacial Surgery: An In Vivo Study
Abstract
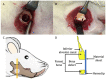
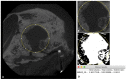
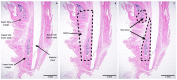
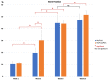
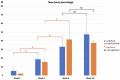
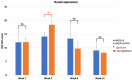
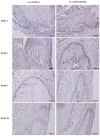
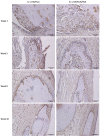
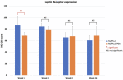
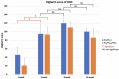
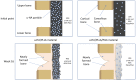
Uncalcined/unsintered hydroxyapatite and poly-l-lactide-co-glycolide (u-HA/PLLA/PGA) is a new bioresorbable nanomaterial with superior characteristics compared with current bioresorbable materials, including appropriate mechanical properties, outstanding bioactive/osteoconductive features, and remarkably shorter resorption time. Nevertheless, the bone regeneration characteristics of this nanomaterial have not been evaluated in maxillofacial reconstructive surgery. In this study, we used a rat mandible model to assess the bone regeneration ability of u-HA/PLLA/PGA material, compared with uncalcined/unsintered hydroxyapatite and poly-l-lactide acid (u-HA/PLLA) material, which has demonstrated excellent bone regenerative ability. A 4-mm-diameter defect was created at the mandibular angle area in 28 Sprague Dawley male rats. The rats were divided into three groups: u-HA/PLLA/PGA (u-HA/PLLA/PGA graft + defect), u-HA/PLLA (u-HA/PLLA graft + defect), and sham control (defect alone). At 1, 3, 8, and 16 weeks after surgeries, the rats were sacrificed and assessed by micro-computed tomography, histological analysis with hematoxylin and eosin staining, and immunohistochemical analyses. The results confirmed that the accelerated bone bioactive/regenerative osteoconduction of u-HA/PLLA/PGA was comparable with that of u-HA/PLLA in the rat mandible model. Furthermore, this new regenerative nanomaterial was able to more rapidly induce bone formation in the early stage and had great potential for further clinical applications in maxillofacial reconstructive surgery.
Keywords: Runx2; bone regeneration; leptin receptor; osteocalcin; osteoconductivity; poly-l-lactic acid; poly-l-lactide-co-glycolide; uncalcined/unsintered hydroxyapatite.
Conflict of interest statement
The authors have no conflict of interest to declare.
Figures






References
-
- Tschakaloff A., Losken H.W., Lalikos J., Link J., Mooney M.P., von Oepen R., Michaeli W., Losken A. Experimental studies of DL-polylactic acid biodegradable plates and screws in rabbits: Computed tomography and molecular weight loss. J. Craniofac. Surg. 1993;4:223–227. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

