Betulinic Acid Protects DOX-Triggered Cardiomyocyte Hypertrophy Response through the GATA-4/Calcineurin/NFAT Pathway
- PMID: 33374365
- PMCID: PMC7795060
- DOI: 10.3390/molecules26010053
Betulinic Acid Protects DOX-Triggered Cardiomyocyte Hypertrophy Response through the GATA-4/Calcineurin/NFAT Pathway
Abstract
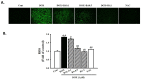
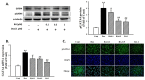
Cardiac hypertrophy is a major risk factor for heart failure and leads to cardiovascular morbidity and mortality. Doxorubicin (DOX) is regarded as one of the most potent anthracycline antibiotic agents; however, its clinical usage has some limitations because it has serious cardiotoxic side effects such as dilated cardiomyopathy and congestive heart failure. Betulinic acid (BA) is a pentacyclic-cyclic lupane-type triterpene that has been reported to have anti-bacterial, anti-inflammatory, anti-vascular neogenesis, and anti-fibrotic effects. However, there is no study about its direct effect on DOX induced cardiac hypertrophy and apoptosis. The present study aims to investigate the effect of BA on DOX-induced cardiomyocyte hypertrophy and apoptosis in vitro in H9c2 cells. The H9c2 cells were stimulated with DOX (1 µM) in the presence or absence of BA (0.1-1 μM) and incubated for 24 h. The results of the present study indicated that DOX induces the increase cell surface area and the upregulation of hypertrophy markers including atrial natriuretic peptide (ANP), B-type natriuretic peptide (BNP), beta-myosin heavy chain (β-MHC), and Myosin Light Chain-2 (MLC2) in H9c2 cells. However, the pathological hypertrophic responses were downregulated after BA treatment. Moreover, phosphorylation of JNK, ERK, and p38 in DOX treated H9c2 cells was blocked by BA. As a result of measuring the change in ROS generation using DCF-DA, BA significantly inhibited DOX-induced the production of intracellular reactive oxygen species (ROS) when BA was treated at a concentration of over 0.1 µM. DOX-induced activation of GATA-4 and calcineurin/NFAT-3 signaling pathway were remarkably improved by pre-treating of BA to H9c2 cells. In addition, BA treatment significantly reduced DOX-induced cell apoptosis and protein expression levels of Bax and cleaved caspase-3/-9, while the expression of Bcl-2 was increased by BA. Therefore, BA can be a potential treatment for cardiomyocyte hypertrophy and apoptosis that lead to sudden heart failure.
Keywords: apoptosis; betulinic acid; cardiomyocyte hypertrophy; doxorubicin; heart failure.
Conflict of interest statement
All authors declare that they have no potential conflicts of interest concerning this article.
Figures





References
-
- Yanti O., Carlo G.T., Kathleen L.G., Janssens S., Crijns H.J., Moens A.L. Doxorubicin-induced cardiomyopathy: From molecular mechanisms to therapeutic strategies. J. Mol. Cell. Cardiol. 2012;52:1213–1225. - PubMed
-
- Armenian S.H., Lacchetti C., Lenihan D. Prevention and monitoring of cardiac dysfunction in survivors of adult cancers: American Society of Clinical Oncology Clinical Practice Guideline. J. Oncol. Pract. 2017;35:893–911. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

