Progress of Cancer Nanotechnology as Diagnostics, Therapeutics, and Theranostics Nanomedicine: Preclinical Promise and Translational Challenges
- PMID: 33374391
- PMCID: PMC7823416
- DOI: 10.3390/pharmaceutics13010024
Progress of Cancer Nanotechnology as Diagnostics, Therapeutics, and Theranostics Nanomedicine: Preclinical Promise and Translational Challenges
Abstract
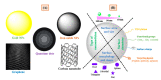
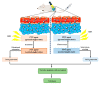
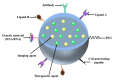
Early detection, right therapeutic intervention, and simultaneous effectiveness mapping are considered the critical factors in successful cancer therapy. Nevertheless, these factors experience the limitations of conventional cancer diagnostics and therapeutics delivery approaches. Along with providing the targeted therapeutics delivery, advances in nanomedicines have allowed the combination of therapy and diagnostics in a single system (called cancer theranostics). This paper discusses the progress in the pre-clinical and clinical development of therapeutics, diagnostics, and theranostics cancer nanomedicines. It has been well evident that compared to the overabundance of works that claimed success in pre-clinical studies, merely 15 and around 75 cancer nanomedicines are approved, and currently under clinical trials, respectively. Thus, we also brief the critical bottlenecks in the successful clinical translation of cancer nanomedicines.
Keywords: and targeted cancer chemotherapy; cancer nanomedicines; diagnostics; metallic nanoparticles; theranostics.
Conflict of interest statement
Sohail Akhter is from Teva Pharmaceutical Industries Ltd. The Company had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results. The authors declare no conflict of interest and the views and Opinions expressed in this article are his personal.
Figures



References
-
- [(accessed on 15 November 2020)]; Available online: https://www.who.int/news-room/fact-sheets/detail/cancer.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

