A Novel Formulation of Glucose-Sparing Peritoneal Dialysis Solutions with l-Carnitine Improves Biocompatibility on Human Mesothelial Cells
- PMID: 33374405
- PMCID: PMC7795315
- DOI: 10.3390/ijms22010123
A Novel Formulation of Glucose-Sparing Peritoneal Dialysis Solutions with l-Carnitine Improves Biocompatibility on Human Mesothelial Cells
Abstract
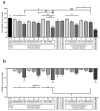
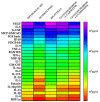
The main reason why peritoneal dialysis (PD) still has limited use in the management of patients with end-stage renal disease (ESRD) lies in the fact that the currently used glucose-based PD solutions are not completely biocompatible and determine, over time, the degeneration of the peritoneal membrane (PM) and consequent loss of ultrafiltration (UF). Here we evaluated the biocompatibility of a novel formulation of dialytic solutions, in which a substantial amount of glucose is replaced by two osmometabolic agents, xylitol and l-carnitine. The effect of this novel formulation on cell viability, the integrity of the mesothelial barrier and secretion of pro-inflammatory cytokines was evaluated on human mesothelial cells grown on cell culture inserts and exposed to the PD solution only at the apical side, mimicking the condition of a PD dwell. The results were compared to those obtained after exposure to a panel of dialytic solutions commonly used in clinical practice. We report here compelling evidence that this novel formulation shows better performance in terms of higher cell viability, better preservation of the integrity of the mesothelial layer and reduced release of pro-inflammatory cytokines. This new formulation could represent a step forward towards obtaining PD solutions with high biocompatibility.
Keywords: glucose-sparing; l-carnitine; mesothelium; peritoneal dialysis; peritoneal dialysis solution; xylitol.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures

Similar articles
-
A New Peritoneal Dialysis Solution Containing L-Carnitine and Xylitol for Patients on Continuous Ambulatory Peritoneal Dialysis: First Clinical Experience.Toxins (Basel). 2021 Feb 24;13(3):174. doi: 10.3390/toxins13030174. Toxins (Basel). 2021. PMID: 33668249 Free PMC article. Clinical Trial.
-
The in vitro biocompatibility performance of a 25 mmol/L bicarbonate/10 mmol/L lactate-buffered peritoneal dialysis fluid.Kidney Int Suppl. 2003 Dec;(88):S94-9. Kidney Int Suppl. 2003. PMID: 14870882
-
Studying the effects of new peritoneal dialysis solutions on the peritoneum.Perit Dial Int. 2007 Jun;27 Suppl 2:S87-93. Perit Dial Int. 2007. PMID: 17556337 Review.
-
Biological Effects of XyloCore, a Glucose Sparing PD Solution, on Mesothelial Cells: Focus on Mesothelial-Mesenchymal Transition, Inflammation and Angiogenesis.Nutrients. 2021 Jun 30;13(7):2282. doi: 10.3390/nu13072282. Nutrients. 2021. PMID: 34209455 Free PMC article.
-
The application of animal models to study the biocompatibility of bicarbonate-buffered peritoneal dialysis solutions.Kidney Int Suppl. 2003 Dec;(88):S75-83. doi: 10.1046/j.1523-1755.2003.08808.x. Kidney Int Suppl. 2003. PMID: 14870880 Review.
Cited by
-
Sweeteners: erythritol, xylitol and cardiovascular risk-friend or foe?Cardiovasc Res. 2025 Aug 14;121(9):1319-1329. doi: 10.1093/cvr/cvaf091. Cardiovasc Res. 2025. PMID: 40444390 Free PMC article. Review.
-
Proteomics and Extracellular Vesicles as Novel Biomarker Sources in Peritoneal Dialysis in Children.Int J Mol Sci. 2022 May 18;23(10):5655. doi: 10.3390/ijms23105655. Int J Mol Sci. 2022. PMID: 35628461 Free PMC article. Review.
-
Human peritoneal tight junction, transporter and channel expression in health and kidney failure, and associated solute transport.Sci Rep. 2023 Oct 13;13(1):17429. doi: 10.1038/s41598-023-44466-z. Sci Rep. 2023. PMID: 37833387 Free PMC article.
-
Mechanisms of Peritoneal Fibrosis: Focus on Immune Cells-Peritoneal Stroma Interactions.Front Immunol. 2021 Mar 29;12:607204. doi: 10.3389/fimmu.2021.607204. eCollection 2021. Front Immunol. 2021. PMID: 33854496 Free PMC article.
-
Volume-Independent Sodium Toxicity in Peritoneal Dialysis: New Insights from Bench to Bed.Int J Mol Sci. 2021 Nov 26;22(23):12804. doi: 10.3390/ijms222312804. Int J Mol Sci. 2021. PMID: 34884617 Free PMC article. Review.
References
-
- Pereira B., Sayegh M., Blake P. In: Chronic Kidney Disease, Dialysis, and Transplantation: Companion to Brenner and Rector’s the Kidney. 2nd ed. Pereira B.J.G., Sayegh M.H., Blake P., editors. Elsevier Saunders; Philadelphia, PA, USA: 2004.
-
- Kramer A., Pippias M., Noordzij M., Stel V.S., Afentakis N., Ambühl P.M., Andrusev A.M., Fuster E.A., Arribas Monzón F.E., Åsberg A., et al. The European Renal Association—European Dialysis and Transplant Association (ERA-EDTA) Registry Annual Report 2015: A summary. Clin. Kidney J. 2018;11:108–122. doi: 10.1093/ckj/sfx149. - DOI - PMC - PubMed

