In Vitro and In Vivo Models of Non-Alcoholic Fatty Liver Disease: A Critical Appraisal
- PMID: 33374435
- PMCID: PMC7794936
- DOI: 10.3390/jcm10010036
In Vitro and In Vivo Models of Non-Alcoholic Fatty Liver Disease: A Critical Appraisal
Abstract
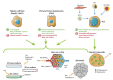
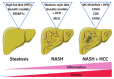
Non-alcoholic fatty liver disease (NAFLD), including non-alcoholic fatty liver (NAFL) and non-alcoholic steatohepatitis (NASH), represents the hepatic manifestation of obesity and metabolic syndrome. Due to the spread of the obesity epidemic, NAFLD is becoming the most common chronic liver disease and one of the principal indications for liver transplantation. However, no pharmacological treatment is currently approved to prevent the outbreak of NASH, which leads to fibrosis and cirrhosis. Preclinical research is required to improve our knowledge of NAFLD physiopathology and to identify new therapeutic targets. In the present review, we summarize advances in NAFLD preclinical models from cellular models, including new bioengineered platforms, to in vivo models, with a particular focus on genetic and dietary mouse models. We aim to discuss the advantages and limits of these different models.
Keywords: NAFLD; NASH; cell culture; liver-on-a-chip; mouse model; organoids; spheroids.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- European Association for the Study of the Liver (EASL) European Association for the Study of Diabetes (EASD) European Association for the Study of Obesity (EASO) EASL–EASD–EASO Clinical Practice Guidelines for the Management of Non-Alcoholic Fatty Liver Disease. J. Hepatol. 2016;64:1388–1402. doi: 10.1016/j.jhep.2015.11.004. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

