Larvicidal Activity of Cinnamic Acid Derivatives: Investigating Alternative Products for Aedes aegypti L. Control
- PMID: 33374484
- PMCID: PMC7796249
- DOI: 10.3390/molecules26010061
Larvicidal Activity of Cinnamic Acid Derivatives: Investigating Alternative Products for Aedes aegypti L. Control
Abstract
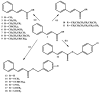
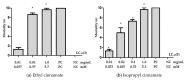
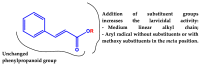
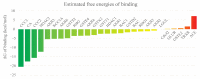
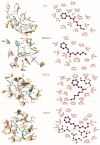
The mosquito Aedes aegypti transmits the virus that causes dengue, yellow fever, Zika and Chikungunya viruses, and in several regions of the planet represents a vector of great clinical importance. In terms of mortality and morbidity, infections caused by Ae. aegypti are among the most serious arthropod transmitted viral diseases. The present study investigated the larvicidal potential of seventeen cinnamic acid derivatives against fourth stage Ae. aegypti larvae. The larvicide assays were performed using larval mortality rates to determine lethal concentration (LC50). Compounds containing the medium alkyl chains butyl cinnamate (7) and pentyl cinnamate (8) presented excellent larvicidal activity with LC50 values of around 0.21-0.17 mM, respectively. While among the derivatives with aryl substituents, the best LC50 result was 0.55 mM for benzyl cinnamate (13). The tested derivatives were natural compounds and in pharmacology and antiparasitic studies, many have been evaluated using biological models for environmental and toxicological safety. Molecular modeling analyses suggest that the larvicidal activity of these compounds might be due to a multi-target mechanism of action involving inhibition of a carbonic anhydrase (CA), a histone deacetylase (HDAC2), and two sodium-dependent cation-chloride co-transporters (CCC2 e CCC3).
Keywords: Chikungunya; Zika; cinnamic ester; dengue; larvicidal activity; medicinal plants; mosquitoes; natural products; yellow fever.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Coelho A.A.M., De Paula J.E., Espíndola L.S. Atividade larvicida de extratos vegetais sobre Aedes aegypti (L.) (Diptera: Culicidae), em condições de laboratório. BioAssay. 2009;4:1–6. doi: 10.14295/BA.v4.0.22. - DOI
-
- Nunes F.C., Leite J.A., Oliveira L.H.G., Sousa P.A.P.S., Menezes M.C., Moraes J.P.S., Mascarenhas S.R., Braga V.A. The larvicidal activity of Agave sisalana against L4 larvae of Aedes aegypti is mediated by internal necrosis and inhibition of nitric oxide production. Parasitol. Res. 2015;114:543–549. doi: 10.1007/s00436-014-4216-y. - DOI - PubMed
-
- Simas N.K., Lima E.C., Conceição S.R., Kuster R.M., de Oliveira Filho A.M. Produtos naturais para o controle da transmissão da dengue – atividade larvicida de Myroxylon balsamum (óleo vermelho) e de terpenóides e fenilpropanóides. Quím. Nova. 2004;27:46–49. doi: 10.1590/S0100-40422004000100009. - DOI
-
- WHO Dengue and Severe Dengue. [(accessed on 17 September 2020)];2020 Available online: https://www.who.int/news-room/fact-sheets/detail/dengue-and-severe-dengue.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

