Fluctuating NMDA Receptor Subunit Levels in Perirhinal Cortex Relate to Their Dynamic Roles in Object Memory Destabilization and Reconsolidation
- PMID: 33374645
- PMCID: PMC7793502
- DOI: 10.3390/ijms22010067
Fluctuating NMDA Receptor Subunit Levels in Perirhinal Cortex Relate to Their Dynamic Roles in Object Memory Destabilization and Reconsolidation
Abstract
Reminder cues can destabilize consolidated memories, rendering them modifiable before they return to a stable state through the process of reconsolidation. Older and stronger memories resist this process and require the presentation of reminders along with salient novel information in order to destabilize. Previously, we demonstrated in rats that novelty-induced object memory destabilization requires acetylcholine (ACh) activity at M1 muscarinic receptors. Other research predominantly has focused on glutamate, which modulates fear memory destabilization and reconsolidation through GluN2B- and GluN2A-containing NMDARs, respectively. In the current study, we demonstrate the same dissociable roles of GluN2B- and N2A-containing NMDARs in perirhinal cortex (PRh) for object memory destabilization and reconsolidation when boundary conditions are absent. However, neither GluN2 receptor subtype was required for novelty-induced destabilization of remote, resistant memories. Furthermore, GluN2B and GluN2A subunit proteins were upregulated selectively in PRh 24 h after learning, but returned to baseline by 48 h, suggesting that NMDARs, unlike muscarinic receptors, have only a temporary role in object memory destabilization. Indeed, activation of M1 receptors in PRh at the time of reactivation effectively destabilized remote memories despite inhibition of GluN2B-containing NMDARs. These findings suggest that cholinergic activity at M1 receptors overrides boundary conditions to destabilize resistant memories when other established mechanisms are insufficient.
Keywords: acetylcholine; boundary conditions; destabilization; glutamate; memory; reconsolidation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
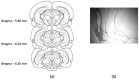

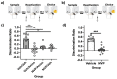
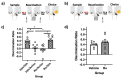

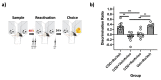

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

