TCR Recognition of Peptide-MHC-I: Rule Makers and Breakers
- PMID: 33374673
- PMCID: PMC7793522
- DOI: 10.3390/ijms22010068
TCR Recognition of Peptide-MHC-I: Rule Makers and Breakers
Abstract
T cells are a critical part of the adaptive immune system that are able to distinguish between healthy and unhealthy cells. Upon recognition of protein fragments (peptides), activated T cells will contribute to the immune response and help clear infection. The major histocompatibility complex (MHC) molecules, or human leukocyte antigens (HLA) in humans, bind these peptides to present them to T cells that recognise them with their surface T cell receptors (TCR). This recognition event is the first step that leads to T cell activation, and in turn can dictate disease outcomes. The visualisation of TCR interaction with pMHC using structural biology has been crucial in understanding this key event, unravelling the parameters that drive this interaction and their impact on the immune response. The last five years has been the most productive within the field, wherein half of current unique TCR-pMHC-I structures to date were determined within this time. Here, we review the new insights learned from these recent TCR-pMHC-I structures and their impact on T cell activation.
Keywords: MHC class I; TCR binding; human leukocyte antigen (HLA); peptide antigens; αβ TCR; γδ TCR; δβ TCR.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
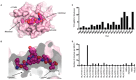


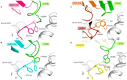


References
-
- McCluskey J., Gras S., Bharadwaj M., Kjer-Nielsen L. The HLA Complex in Biology and Medicine: A Resource Book. Jaypee Brothers Medical Publishing; New Delhi, India: 2010. HLA Molecules of the Major Histocompatibility Complex; p. 86.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

