Intra-Articular Injection of (-)-Epigallocatechin 3-Gallate to Attenuate Articular Cartilage Degeneration by Enhancing Autophagy in a Post-Traumatic Osteoarthritis Rat Model
- PMID: 33374730
- PMCID: PMC7824012
- DOI: 10.3390/antiox10010008
Intra-Articular Injection of (-)-Epigallocatechin 3-Gallate to Attenuate Articular Cartilage Degeneration by Enhancing Autophagy in a Post-Traumatic Osteoarthritis Rat Model
Abstract
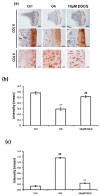
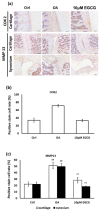
(-)-Epigallocatechin 3-gallate (EGCG) is the main active green tea catechin and has a wide variety of benefits for health. Post-traumatic osteoarthritis (PTOA) occurs as a consequence of joint injuries that commonly happen in the young population. In this study, we investigated the effects of EGCG on PTOA prevention by using the anterior cruciate ligament transection (ACLT)-OA model and further investigated the roles of autophagy in OA treatment. Our results showed that intra-articular injection of EGCG significantly improved the functional performances and decreased cartilage degradation. EGCG treatment attenuated the inflammation on synovial tissue and cartilage through less immunostained cyclooxygenase-2 and matrix metalloproteinase-13. We further noted EGCG may modulate the chondrocyte apoptosis by activation of the cytoprotective autophagy through reducing the expression of the mTOR and enhancing the expression of microtubule-associated protein light chain 3, beclin-1, and p62. In conclusion, intra-articular injection of EGCG after ACL injury inhibited the joint inflammation and cartilage degradation, thereby increasing joint function. EGCG treatment also reduced the chondrocyte apoptosis, possibly by activating autophagy. These findings suggested that EGCG may be a potential disease-modifying drug for preventing OA progression.
Keywords: (-)-epigallocatechin 3-gallate (EGCG); apoptosis; autophagy; cartilage; mTOR; post-traumatic osteoarthritis.
Conflict of interest statement
The authors declare that there is no conflict of interest. The funders had no role in the study design, data collection or analysis; the decision to publish; or the preparation of the manuscript.
Figures





References
-
- Tawonsawatruk T., Sriwatananukulkit O., Himakhun W., Hemstapat W. Comparison of pain behaviour and osteoarthritis progression between anterior cruciate ligament transection and osteochondral injury in rat models. Bone Jt. Res. 2018;7:244–251. doi: 10.1302/2046-3758.73.BJR-2017-0121.R2. - DOI - PMC - PubMed
-
- Buckwalter J.A., Mankin H.J., Grodzinsky A.J. Articular cartilage and osteoarthritis. Instr. Course Lect. 2005;54:465–480. - PubMed
Grants and funding
- NHRI-EX101-9935EI/National Health Research Institute
- KMUH-107-7R54/Kaohsiung Medical University Hospital
- KMU-TC108A02-1, NCTUKMU108-BIO-04, NPUST-KMU-109-P002 and KMU-DK10500/Kaohsiung Medical University
- KMTTH107-26/Kaohsiung Municipal Ta-Tung Hospital
- MOST 108-2314-B-037 -059 -MY3/Minister of Science and Technology of Taiwan
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

