Resources, Production Scales and Time Required for Producing RNA Vaccines for the Global Pandemic Demand
- PMID: 33374802
- PMCID: PMC7824664
- DOI: 10.3390/vaccines9010003
Resources, Production Scales and Time Required for Producing RNA Vaccines for the Global Pandemic Demand
Erratum in
-
Correction: Kis, Z. et al. Resources, Production Scales and Time Required for Producing RNA Vaccines for the Global Pandemic Demand. Vaccines 2021, 9, 3.Vaccines (Basel). 2021 Mar 1;9(3):205. doi: 10.3390/vaccines9030205. Vaccines (Basel). 2021. PMID: 33804590 Free PMC article.
Abstract
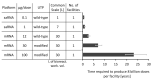
To overcome pandemics, such as COVID-19, vaccines are urgently needed at very high volumes. Here we assess the techno-economic feasibility of producing RNA vaccines for the demand associated with a global vaccination campaign. Production process performance is assessed for three messenger RNA (mRNA) and one self-amplifying RNA (saRNA) vaccines, all currently under clinical development, as well as for a hypothetical next-generation saRNA vaccine. The impact of key process design and operation uncertainties on the performance of the production process was assessed. The RNA vaccine drug substance (DS) production rates, volumes and costs are mostly impacted by the RNA amount per vaccine dose and to a lesser extent by the scale and titre in the production process. The resources, production scale and speed required to meet global demand vary substantially in function of the RNA amount per dose. For lower dose saRNA vaccines, global demand can be met using a production process at a scale of below 10 L bioreactor working volume. Consequently, these small-scale processes require a low amount of resources to set up and operate. RNA DS production can be faster than fill-to-finish into multidose vials; hence the latter may constitute a bottleneck.
Keywords: COVID-19; RNA vaccines; mRNA vaccines; pandemic-response vaccine production; production process modelling; saRNA vaccines; techno-economic analysis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- World Health Organization DRAFT Landscape of COVID-19 Candidate Vaccines. [(accessed on 12 November 2020)];2020 Available online: https://www.who.int/publications/m/item/draft-landscape-of-covid-19-cand....
-
- Dolgin E. COVID-19 Vaccines Poised for Launch, but Impact on Pandemic Unclear. [(accessed on 26 November 2020)];Nat. Biotechnol. 2020 doi: 10.1038/d41587-020-00022-y. Available online: https://www.nature.com/articles/d41587-020-00022-y. - DOI - PubMed
-
- McKay P.F., Hu K., Blakney A.K., Samnuan K., Brown J.C., Penn R., Zhou J., Bouton C.R., Rogers P., Polra K., et al. Self-amplifying RNA SARS-CoV-2 lipid nanoparticle vaccine candidate induces high neutralizing antibody titers in mice. Nat. Commun. 2020;11:3523. doi: 10.1038/s41467-020-17409-9. - DOI - PMC - PubMed
-
- Vogel A.B., Lambert L., Kinnear E., Busse D., Erbar S., Reuter K.C., Wicke L., Perkovic M., Beissert T., Haas H., et al. Self-Amplifying RNA Vaccines Give Equivalent Protection against Influenza to mRNA Vaccines but at Much Lower Doses. Mol. Ther. 2018;26:26446–26455. doi: 10.1016/j.ymthe.2017.11.017. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

