The ER Stress/UPR Axis in Chronic Obstructive Pulmonary Disease and Idiopathic Pulmonary Fibrosis
- PMID: 33374938
- PMCID: PMC7821926
- DOI: 10.3390/life11010001
The ER Stress/UPR Axis in Chronic Obstructive Pulmonary Disease and Idiopathic Pulmonary Fibrosis
Abstract
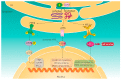
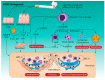
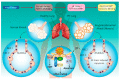
Cellular protein homeostasis in the lungs is constantly disrupted by recurrent exposure to various external and internal stressors, which may cause considerable protein secretion pressure on the endoplasmic reticulum (ER), resulting in the survival and differentiation of these cell types to meet the increased functional demands. Cells are able to induce a highly conserved adaptive mechanism, known as the unfolded protein response (UPR), to manage such stresses. UPR dysregulation and ER stress are involved in numerous human illnesses, such as metabolic syndrome, fibrotic diseases, and neurodegeneration, and cancer. Therefore, effective and specific compounds targeting the UPR pathway are being considered as potential therapies. This review focuses on the impact of both external and internal stressors on the ER in idiopathic pulmonary fibrosis (IPF) and chronic obstructive pulmonary disease (COPD) and discusses the role of the UPR signaling pathway activation in the control of cellular damage and specifically highlights the potential involvement of non-coding RNAs in COPD. Summaries of pathogenic mechanisms associated with the ER stress/UPR axis contributing to IPF and COPD, and promising pharmacological intervention strategies, are also presented.
Keywords: UPR; autophagy; endoplasmic reticulum; fibrosis; lung disease; non-coding RNA; tissue remodeling.
Conflict of interest statement
The authors have no conflict of interest.
Figures