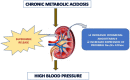
Chronic Metabolic Acidosis Elicits Hypertension via Upregulation of Intrarenal Angiotensin II and Induction of Oxidative Stress
- PMID: 33374943
- PMCID: PMC7821948
- DOI: 10.3390/antiox10010002
Chronic Metabolic Acidosis Elicits Hypertension via Upregulation of Intrarenal Angiotensin II and Induction of Oxidative Stress
Abstract
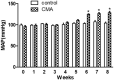
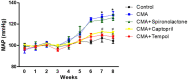
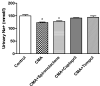
Chronic metabolic acidosis (CMA) can be a consequence of persistent hypertension but could potentially play a role in invoking hypertension. Currently, there is a scarcity of studies examining the outcome of induced chronic acidosis on blood pressure regulation. This study investigates CMA as a cause of hypertension. Chronic acidosis was induced in Sprague Dawley rats (100-150 g) by providing a weak acid solution of 0.28 M ammonium chloride (NH4Cl) in tap water for 8 weeks. To determine whether the rats were acidotic, blood pH was measured, while blood pressure (BP) was monitored by tail-cuff plethysmography weekly. Rats were divided into five groups: control, CMA, CMA ± spironolactone, captopril, and tempol. Serum sodium and potassium; renal interstitial fluid (for Angiotensin II concentration); and kidney proximal tubules (for Na+/K+ ATPase- α1 concentration) were analyzed. Reactive oxygen species (ROS) were detected in renal cortical homogenates using electron paramagnetic resonance (EPR). In the CMA rats, a sustained elevation in mean arterial pressure (MAP) associated with a significant decrease in blood pH was observed compared to that of control over the 8 weeks. A significant decrease in MAP was observed in acidotic rats treated with captopril/tempol, whereas spironolactone treatment caused no decrease in MAP as compared to that of the CMA group. The interstitial angiotensin II was increased in the CMA group but decreased in the CMA with captopril and tempol groups. In addition, the urinary sodium was decreased, and the serum sodium levels increased significantly in the CMA groups as compared to that of control. However, the acidotic groups with captopril and tempol showed reduced levels of serum sodium and an elevation in urinary sodium as compared to that of the CMA group. In addition, there was a significant increase in plasma renin and no change in plasma aldosterone in the CMA group with no significant differences in plasma renin or aldosterone observed during spironolactone, captopril, or tempol treatments. The increased expression of Na+/K+ ATPase-α1 in the CMA group suggests that active transport of Na+ to the blood could be causative of the observed hypertension. Furthermore, the EPR analysis confirmed an elevation in superoxide (O2-) radical levels in the CMA group, but the tempol/captopril treated acidotic groups showed less (O2-) compared to that of either the CMA group or control. Taken together, our data suggest that induction of CMA could potentially be causative of hypertension, while the mechanisms underlying the increased BP could be through the activation of intrarenal Ang II and induction of oxidative stress.
Keywords: angiotensin II; hypertension; metabolic acidosis.
Conflict of interest statement
The authors declare there is no conflict of interest regarding the publication of this paper. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures









References
-
- Foëx P., Sear J. Hypertension: Pathophysiology and treatment. Contin. Educ. Anaesth. Crit. Care Pain. 2004;4:71–75. doi: 10.1093/bjaceaccp/mkh020. - DOI
-
- Bhagavan N.V., Ha C.E. Essentials of Medical Biochemistry. 2nd ed. Elsevier Inc.; Amsterdam, The Netherlands: 2015. p. 752.
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

