Hydrophilic Biocompatible Poly(Acrylic Acid-co-Maleic Acid) Polymer as a Surface-Coating Ligand of Ultrasmall Gd2O3 Nanoparticles to Obtain a High r1 Value and T1 MR Images
- PMID: 33375089
- PMCID: PMC7822180
- DOI: 10.3390/diagnostics11010002
Hydrophilic Biocompatible Poly(Acrylic Acid-co-Maleic Acid) Polymer as a Surface-Coating Ligand of Ultrasmall Gd2O3 Nanoparticles to Obtain a High r1 Value and T1 MR Images
Abstract
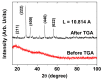
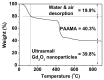
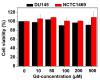
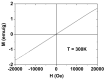
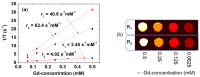
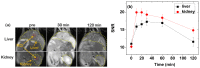
The water proton spin relaxivity, colloidal stability, and biocompatibility of nanoparticle-based magnetic resonance imaging (MRI) contrast agents depend on the surface-coating ligands. Here, poly(acrylic acid-co-maleic acid) (PAAMA) (Mw = ~3000 amu) is explored as a surface-coating ligand of ultrasmall gadolinium oxide (Gd2O3) nanoparticles. Owing to the numerous carboxylic groups in PAAMA, which allow its strong conjugation with the nanoparticle surfaces and the attraction of abundant water molecules to the nanoparticles, the synthesized PAAMA-coated ultrasmall Gd2O3 nanoparticles (davg = 1.8 nm and aavg = 9.0 nm) exhibit excellent colloidal stability, extremely low cellular toxicity, and a high longitudinal water proton spin relaxivity (r1) of 40.6 s-1mM-1 (r2/r1 = 1.56, where r2 = transverse water proton spin relaxivity), which is approximately 10 times higher than those of commercial molecular contrast agents. The effectiveness of PAAMA-coated ultrasmall Gd2O3 nanoparticles as a T1 MRI contrast agent is confirmed by the high positive contrast enhancements of the in vivo T1 MR images at the 3.0 T MR field.
Keywords: biocompatibility; colloidal stability; magnetic resonance imaging agent; poly(acrylic acid-co-maleic acid); relaxivity; ultrasmall Gd2O3 nanoparticle.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
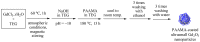




References
-
- Hashemi R.H., Bradley W.G., Lisanti C.J. MRI the Basics. Lippincott Williams & Wilkins; New York, NY, USA: 2004.
-
- Lauffer R.B. Paramagnetic metal complexes as water proton relaxation agents for NMR imaging: Theory and design. Chem. Rev. 1987;87:901–927. doi: 10.1021/cr00081a003. - DOI
-
- Greenwood N.N., Earnshaw A. Chemistry of the Elements. Butterworth-Heinemann; Oxford, UK: 1997. p. 1243.
Grants and funding
LinkOut - more resources
Full Text Sources

