Molecules, Mechanisms, and Disorders of Self-Domestication: Keys for Understanding Emotional and Social Communication from an Evolutionary Perspective
- PMID: 33375093
- PMCID: PMC7822183
- DOI: 10.3390/biom11010002
Molecules, Mechanisms, and Disorders of Self-Domestication: Keys for Understanding Emotional and Social Communication from an Evolutionary Perspective
Abstract
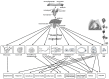
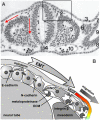
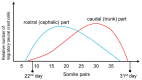
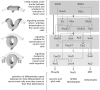
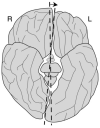
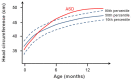
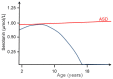
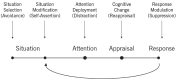
The neural crest hypothesis states that the phenotypic features of the domestication syndrome are due to a reduced number or disruption of neural crest cells (NCCs) migration, as these cells differentiate at their final destinations and proliferate into different tissues whose activity is reduced by domestication. Comparing the phenotypic characteristics of modern and prehistoric man, it is clear that during their recent evolutionary past, humans also went through a process of self-domestication with a simultaneous prolongation of the period of socialization. This has led to the development of social abilities and skills, especially language, as well as neoteny. Disorders of neural crest cell development and migration lead to many different conditions such as Waardenburg syndrome, Hirschsprung disease, fetal alcohol syndrome, DiGeorge and Treacher-Collins syndrome, for which the mechanisms are already relatively well-known. However, for others, such as Williams-Beuren syndrome and schizophrenia that have the characteristics of hyperdomestication, and autism spectrum disorders, and 7dupASD syndrome that have the characteristics of hypodomestication, much less is known. Thus, deciphering the biological determinants of disordered self-domestication has great potential for elucidating the normal and disturbed ontogenesis of humans, as well as for the understanding of evolution of mammals in general.
Keywords: chemoattractants; chemorepellents; epithelial-mesenchymal transition (EMT); extracellular matrix molecules; fibroblast growth factor (FGF); methyl-CpG-binding protein 2 (MeCP2); neural crest cells (NCCs); self-domestication; thyroid hormones; vascular endothelial growth factor (VEGF).
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Schizophrenia and Human Self-Domestication: An Evolutionary Linguistics Approach.Brain Behav Evol. 2017;89(3):162-184. doi: 10.1159/000468506. Epub 2017 May 3. Brain Behav Evol. 2017. PMID: 28463847
-
Upregulation of the Nr2f1-A830082K12Rik gene pair in murine neural crest cells results in a complex phenotype reminiscent of Waardenburg syndrome type 4.Dis Model Mech. 2016 Nov 1;9(11):1283-1293. doi: 10.1242/dmm.026773. Epub 2016 Sep 1. Dis Model Mech. 2016. PMID: 27585883 Free PMC article.
-
Shared reproductive disruption, not neural crest or tameness, explains the domestication syndrome.Proc Biol Sci. 2023 Mar 29;290(1995):20222464. doi: 10.1098/rspb.2022.2464. Epub 2023 Mar 22. Proc Biol Sci. 2023. PMID: 36946116 Free PMC article. Review.
-
Neurocristopathies: Enigmatic Appearances of Neural Crest Cell-derived Abnormalities.Radiographics. 2019 Nov-Dec;39(7):2085-2102. doi: 10.1148/rg.2019190086. Radiographics. 2019. PMID: 31697622
-
SOX10: 20 years of phenotypic plurality and current understanding of its developmental function.J Med Genet. 2022 Feb;59(2):105-114. doi: 10.1136/jmedgenet-2021-108105. Epub 2021 Oct 19. J Med Genet. 2022. PMID: 34667088 Free PMC article. Review.
Cited by
-
Evolution and dysfunction of human cognitive and social traits: A transcriptional regulation perspective.Evol Hum Sci. 2022 Sep 26;4:e43. doi: 10.1017/ehs.2022.42. eCollection 2022. Evol Hum Sci. 2022. PMID: 37588924 Free PMC article. Review.
-
The neural crest/domestication syndrome hypothesis, explained: reply to Johnsson, Henriksen, and Wright.Genetics. 2021 Aug 26;219(1):iyab098. doi: 10.1093/genetics/iyab098. Genetics. 2021. PMID: 34849912 Free PMC article. No abstract available.
-
Stay social, stay young: a bioanthropological outlook on the processes linking sociality and ageing.Geroscience. 2025 Feb;47(1):721-744. doi: 10.1007/s11357-024-01416-5. Epub 2024 Nov 11. Geroscience. 2025. PMID: 39527178 Free PMC article. Review.
-
DLX5/6 GABAergic Expression Affects Social Vocalization: Implications for Human Evolution.Mol Biol Evol. 2021 Oct 27;38(11):4748-4764. doi: 10.1093/molbev/msab181. Mol Biol Evol. 2021. PMID: 34132815 Free PMC article.
-
Reflections on the Role of Differentiation Processes in Forming Behavioral Phenotypes: Can These Processes Replace the Concepts of Plastic Phenotype and Reversible Plastic Phenotype?Biology (Basel). 2025 Feb 12;14(2):187. doi: 10.3390/biology14020187. Biology (Basel). 2025. PMID: 40001955 Free PMC article.
References
-
- Darwin C. The Variation of Plants and Animals under Domestication. John Murray; London, UK: 1868.
-
- Irving-Pease E.K., Ryan H., Jamieson A., Dimopoulos E.A. Paleogenomics of animal domestication. In: Lindqvist C., Rajora O., editors. Paleogenomics. Population Genomics. Springer; Berlin/Heidelberg, Germany: 2018. pp. 225–272.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

