Bile-Based Cell-Free DNA Analysis Is a Reliable Diagnostic Tool in Pancreatobiliary Cancer
- PMID: 33375555
- PMCID: PMC7818177
- DOI: 10.3390/cancers13010039
Bile-Based Cell-Free DNA Analysis Is a Reliable Diagnostic Tool in Pancreatobiliary Cancer
Abstract
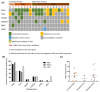
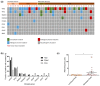
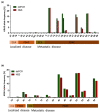
Currently available serum biomarkers for pancreatobiliary cancers lack sensitivity and specificity and ultimate diagnosis still requires invasive procedures for histological confirmation. The detection of tumor-specific genetic aberrations with utilization of cell free DNA (cfDNA) is a less invasive approach than traditional tissue biopsies; however, it has not been implemented into clinical routine. In this study, we investigated bile as a liquid biopsy source in pancreatobiliary cancers and compared its potential as cell-free DNA source to plasma. Blood (n = 37) and bile (n = 21) samples were collected from patients affected by pancreatic ductal adenocarcinoma (PDAC) and extrahepatic cholangiocarcinoma (CCA) or with non-malignant biliary obstructions (blood n = 16; bile n = 21). Panel-based next generation sequencing (NGS) and digital droplet PCR (ddPCR) were applied for tumor mutation profiling. NGS results from matched tumor tissues (n = 29) served as comparison. Sequencing of cfDNA from bile resulted in detection of 96.2% of the pathogenic tumor mutations found in matched tissue samples. On the other hand, only 31.6% of pathogenic tumor mutations found in tissue could be detected in plasma. In a direct comparison, only half of the mutations detected in bile cfDNA were concordantly detected in plasma from the same patients. Panel NGS and ddPCR displayed comparable sensitivity. In conclusion, bile is a suitable source of cfDNA for the diagnosis of pancreatobiliary cancer and performs more reliably than plasma. Although primary diagnosis still requires histologic confirmation, bile-derived cfDNA could offer an alternative if tissue sampling is not feasible and might allow less invasive disease monitoring.
Keywords: ERCP; cell-free DNA; cholangiocarcinoma; liquid biopsy; next generation sequencing; pancreatic cancer.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Groot V.P., van Santvoort H.C., Rombouts S.J.E., Hagendoorn J., Borel Rinkes I.H.M., van Vulpen M., Herman J.M., Wolfgang C.L., Besselink M.G., Molenaar I.Q. Systematic review on the treatment of isolated local recurrence of pancreatic cancer after surgery; re-resection, chemoradiotherapy and SBRT. HPB. 2017;19:83–92. doi: 10.1016/j.hpb.2016.11.001. - DOI - PubMed
-
- Endo I., Gonen M., Yopp A.C., Dalal K.M., Zhou Q., Klimstra D., D’Angelica M., DeMatteo R.P., Fong Y., Schwartz L., et al. Intrahepatic cholangiocarcinoma: Rising frequency, improved survival, and determinants of outcome after resection. Ann. Surg. 2008;248:84–96. doi: 10.1097/SLA.0b013e318176c4d3. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

