Platforms Exploited for SARS-CoV-2 Vaccine Development
- PMID: 33375677
- PMCID: PMC7824029
- DOI: 10.3390/vaccines9010011
Platforms Exploited for SARS-CoV-2 Vaccine Development
Abstract
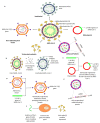
The novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is the only zoonotic-origin coronavirus (CoV) that has reached the pandemic stage. The virus uses its spike (S) glycoprotein to attach to the host cells and initiate a cascade of events that leads to infection. It has sternly affected public health, economy, education, and social behavior around the world. Several scientific and medical communities have mounted concerted efforts to limit this pandemic and the subsequent wave of viral spread by developing preventative and potential vaccines. So far, no medicine or vaccine has been approved to prevent or treat coronavirus disease 2019 (COVID-19). This review describes the latest advances in the development of SARS-CoV-2 vaccines for humans, mainly focusing on the lead candidates in clinical trials. Moreover, we seek to provide both the advantages and the disadvantages of the leading platforms used in current vaccine development, based on past vaccine delivery efforts for non-SARS CoV-2 infections. We also highlight the population groups who should receive a vaccine against COVID-19 in a timely manner to eradicate the pandemic rapidly.
Keywords: Covid-19; SARS-CoV-2; clinical trials; vaccine platforms; vaccines.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

