Extracellular Vesicles from Red Blood Cells and Their Evolving Roles in Health, Coagulopathy and Therapy
- PMID: 33375718
- PMCID: PMC7796437
- DOI: 10.3390/ijms22010153
Extracellular Vesicles from Red Blood Cells and Their Evolving Roles in Health, Coagulopathy and Therapy
Abstract
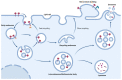
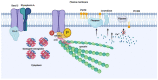
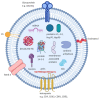
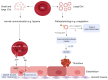
Red blood cells (RBCs) release extracellular vesicles (EVs) including both endosome-derived exosomes and plasma-membrane-derived microvesicles (MVs). RBC-derived EVs (RBCEVs) are secreted during erythropoiesis, physiological cellular aging, disease conditions, and in response to environmental stressors. RBCEVs are enriched in various bioactive molecules that facilitate cell to cell communication and can act as markers of disease. RBCEVs contribute towards physiological adaptive responses to hypoxia as well as pathophysiological progression of diabetes and genetic non-malignant hematologic disease. Moreover, a considerable number of studies focus on the role of EVs from stored RBCs and have evaluated post transfusion consequences associated with their exposure. Interestingly, RBCEVs are important contributors toward coagulopathy in hematological disorders, thus representing a unique evolving area of study that can provide insights into molecular mechanisms that contribute toward dysregulated hemostasis associated with several disease conditions. Relevant work to this point provides a foundation on which to build further studies focused on unraveling the potential roles of RBCEVs in health and disease. In this review, we provide an analysis and summary of RBCEVs biogenesis, composition, and their biological function with a special emphasis on RBCEV pathophysiological contribution to coagulopathy. Further, we consider potential therapeutic applications of RBCEVs.
Keywords: cell-to-cell communication; coagulopathy; exosomes; extracellular vesicles; homeostasis; microparticles; microvesicles; red blood cells.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Horder E. Blood dust or blood granules: A new constituent of the blood? Lancet. 1899;154:1015. doi: 10.1016/S0140-6736(01)59201-6. - DOI
-
- Nguyen D.B., Ly T.B.T., Bernhardt I. Microvesicles Released from Human Red Blood Cells: Properties and Potential Applications. Volume 10 IntechOpen; Rijeka, Croatia: 2017.
-
- Johnstone R.M., Adam M., Hammond J.R., Orr L., Turbide C. Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes) J. Biol. Chem. 1987;262:9412–9420. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

