Phase IB Study of Osimertinib in Combination with Navitoclax in EGFR-mutant NSCLC Following Resistance to Initial EGFR Therapy (ETCTN 9903)
- PMID: 33376097
- PMCID: PMC7976451
- DOI: 10.1158/1078-0432.CCR-20-4084
Phase IB Study of Osimertinib in Combination with Navitoclax in EGFR-mutant NSCLC Following Resistance to Initial EGFR Therapy (ETCTN 9903)
Abstract
Purpose: Osimertinib is an effective therapy in EGFR-mutant non-small cell lung cancer (NSCLC), but resistance invariably develops. Navitoclax is an oral inhibitor of BCL-2/BCL-xL that has exhibited synergy with osimertinib in preclinical models of EGFR-mutant NSCLC. In hematologic malignancies, BCL-2 family inhibitors in combination therapy effectively increase cellular apoptosis and decrease drug resistance.
Patients and methods: This single-arm phase Ib study evaluated safety, tolerability, and feasibility of osimertinib and navitoclax, including dose expansion in T790M-positive patients at the recommended phase II dose (RP2D). Eligible patients had advanced EGFR-mutant NSCLC with prior tyrosine kinase inhibitor exposure. Five dose levels were planned with osimertinib from 40 to 80 mg orally daily and navitoclax from 150 to 325 mg orally daily.
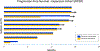
Results: A total of 27 patients were enrolled (18 in the dose-escalation cohort and nine in the dose-expansion cohort): median age 65, 67% female, 48% exon 19 del, and 37% L858R, median one prior line of therapy. The most common adverse events were lymphopenia (37%), fatigue (22%), nausea (22%), and thrombocytopenia (37%). No dose-limiting toxicities were seen in dose-escalation cohort; osimertinib 80 mg, navitoclax 150 mg was chosen as the RP2D. Most patients (78%) received >95% of planned doses through three cycles. In expansion cohort, objective response rate was 100% and median progression-free survival was 16.8 months. A proapoptotic effect from navitoclax was demonstrated by early-onset thrombocytopenia.
Conclusions: Oral combination therapy with navitoclax and osimertinib was safe and feasible at RP2D with clinical efficacy. Early thrombocytopenia was common, supporting an target engagement by navitoclax. Further study of BCL-2/BCL-xL inhibition to enhance osimertinib activity is warranted.
©2020 American Association for Cancer Research.
Figures

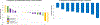

References
-
- Korsmeyer SJ, BCL-2 gene family and the regulation of programmed cell death. Cancer Res, 1999. 59(7 Suppl): p. 1693s–1700s. - PubMed
-
- Korsmeyer SJ, Programmed cell death and the regulation of homeostasis. Harvey Lect, 1999. 95: p. 21–41. - PubMed
-
- Korsmeyer SJ, et al. , Death and survival signals determine active/inactive conformations of pro-apoptotic BAX, BAD, and BID molecules. Cold Spring Harb Symp Quant Biol, 1999. 64: p. 343–50. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

