Multicenter Study of Long-Term Safety of Tolvaptan in Later-Stage Autosomal Dominant Polycystic Kidney Disease
- PMID: 33376102
- PMCID: PMC7792652
- DOI: 10.2215/CJN.10250620
Multicenter Study of Long-Term Safety of Tolvaptan in Later-Stage Autosomal Dominant Polycystic Kidney Disease
Abstract
Background and objectives: Tolvaptan slows kidney function decline in patients with autosomal dominant polycystic kidney disease (ADPKD) at risk of rapid progression. In the 3-year Tolvaptan Efficacy and Safety in Management of ADPKD and Its Outcomes (TEMPO) 3:4, 2-year extension to TEMPO 3:4 (TEMPO 4:4), and 1-year Replicating Evidence of Preserved Renal Function: An Investigation of Tolvaptan Safety and Efficacy in ADPKD (REPRISE) trials, aquaretic adverse events were common. Serum alanine aminotransferase (ALT) or aspartate aminotransferase (AST) elevations occurred in all three studies. Three patients met Hy Law criteria (ALT or AST more than three times and total bilirubin more than two times the upper limit of normal) for severe drug-induced liver injury (two in TEMPO 3:4 and one in TEMPO 4:4). In REPRISE, liver enzyme monitoring frequency was increased to monthly, with no Hy Law cases. A long-term, phase 3 safety study has further characterized tolvaptan safety.
Design, setting, participants, & measurements: Subjects who completed TEMPO 4:4, REPRISE, or other tolvaptan trials could enroll in this prospective, multinational, open-label safety study. Assessments included monthly liver enzyme testing during the first 18 months of tolvaptan exposure and every 3 months thereafter.
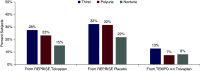
Results: Among 1803 subjects, median tolvaptan exposure during the extension was 651 days (interquartile range, 538-924), and cumulative exposure (extension and previous trials) was ≤11 years. Subjects entering from REPRISE placebo experienced more aquaretic adverse events compared with subjects from TEMPO 4:4 or REPRISE tolvaptan (i.e., patients with prior long-term tolvaptan exposure). Liver enzyme elevations also occurred more frequently in subjects from REPRISE placebo. Percentages experiencing ALT ≥3/≥5/ ≥10/≥20 times the upper limit of normal were 3.2%/2.1%/0.9%/0.7%, respectively, in subjects from REPRISE placebo and 0.6%-1.1%/0.0%-0.1%/0%/0%, respectively, in those from REPRISE tolvaptan and TEMPO 4:4. Percentages experiencing AST ≥3/ ≥5/≥10/≥20 times the upper limit of normal were 6.9%/3.8%/2.3%/0.8%, respectively, in subjects from REPRISE placebo and 0.9%-2.0%/0.0%-1.0%/0%/0%, respectively, in those from REPRISE tolvaptan and TEMPO 4:4. No Hy Law cases occurred.
Conclusions: No new safety signals emerged during this long-term extension. Monthly liver function testing for the first 18 months of treatment appeared to enable effective detection and management of transaminase elevations.
Clinical trial registry name and registration number: Open Label Extension of TEMPO 3:4, NCT02251275.
Keywords: ADPKD; cystic kidney; tolvaptan.
Copyright © 2021 by the American Society of Nephrology.
Figures




Comment in
-
Long-Term Safety of Tolvaptan in ADPKD: Where Do We Stand?Clin J Am Soc Nephrol. 2020 Dec 31;16(1):3-5. doi: 10.2215/CJN.17981120. Epub 2020 Dec 29. Clin J Am Soc Nephrol. 2020. PMID: 33376103 Free PMC article. No abstract available.
References
-
- Spithoven EM, Kramer A, Meijer E, Orskov B, Wanner C, Caskey F, Collart F, Finne P, Fogarty DG, Groothoff JW, Hoitsma A, Nogier MB, Postorino M, Ravani P, Zurriaga O, Jager KJ, Gansevoort RT; ERA-EDTA Registry; EuroCYST Consortium; WGIKD; EuroCYST Consortium; WGIKD: Analysis of data from the ERA-EDTA Registry indicates that conventional treatments for chronic kidney disease do not reduce the need for renal replacement therapy in autosomal dominant polycystic kidney disease. Kidney Int 86: 1244–1252, 2014 - PubMed
-
- van Gastel MDA, Torres VE: Polycystic kidney disease and the vasopressin pathway. Ann Nutr Metab 70[Suppl 1]: 43–50, 2017 - PubMed
-
- Devuyst O, Torres VE: Osmoregulation, vasopressin, and cAMP signaling in autosomal dominant polycystic kidney disease. Curr Opin Nephrol Hypertens 22: 459–470, 2013 - PubMed
-
- Torres VE, Chapman AB, Devuyst O, Gansevoort RT, Grantham JJ, Higashihara E, Perrone RD, Krasa HB, Ouyang J, Czerwiec FS; TEMPO 3:4 Trial Investigators: Tolvaptan in patients with autosomal dominant polycystic kidney disease. N Engl J Med 367: 2407–2418, 2012
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

