A conformation-specific antibody against oligomeric β-amyloid restores neuronal integrity in a mouse model of Alzheimer's disease
- PMID: 33376140
- PMCID: PMC7948963
- DOI: 10.1074/jbc.RA120.015327
A conformation-specific antibody against oligomeric β-amyloid restores neuronal integrity in a mouse model of Alzheimer's disease
Abstract
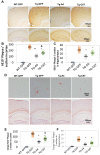
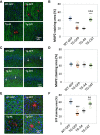
Conformationally distinct aggregates of the amyloid β (Aβ) peptide accumulate in brains of patients with Alzheimer's disease (AD), but the roles of the different aggregates in disease progression are not clear. We previously isolated two single-chain variable domain antibody fragments (scFvs), C6T and A4, that selectively bind different toxic conformational variants of oligomeric Aβ. Here, we utilize these scFvs to localize the presence of these Aβ variants in human AD brain and to demonstrate their potential as therapeutic agents for treating AD. Both A4 and C6T label oligomeric Aβ in extracellular amyloid plaques, whereas C6T also labels intracellular oligomeric Aβ in human AD brain tissue and in an AD mouse model. For therapeutic studies, the A4 and C6T scFvs were expressed in the AD mice by viral infection of liver cells. The scFvs were administered at 2 months of age, and mice sacrificed at 9 months. The scFvs contained a peptide tag to facilitate transport across the blood brain barrier. While treatment with C6T only slightly decreased Aβ deposits and plaque-associated inflammation, it restored neuronal integrity to WT levels, significantly promoted growth of new neurons, and impressively rescued survival rates to WT levels. Treatment with A4 on the other hand significantly decreased Aβ deposits but did not significantly decrease neuroinflammation or promote neuronal integrity, neurogenesis, or survival rate. These results suggest that the specific Aβ conformation targeted in therapeutic applications greatly affects the outcome, and the location of the targeted Aβ variants may also play a critical factor.
Keywords: Alzheimer's disease; neuron; oligomeric beta amyloid; single-chain antibody; transgenic mice.
Copyright © 2021 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest M. Sierks is a cofounder of Studio Biotherapeutics.
Figures




Similar articles
-
Bispecific Antibody Fragment Targeting APP and Inducing α-Site Cleavage Restores Neuronal Health in an Alzheimer's Mouse Model.Mol Neurobiol. 2019 Nov;56(11):7420-7432. doi: 10.1007/s12035-019-1597-z. Epub 2019 Apr 30. Mol Neurobiol. 2019. PMID: 31041656
-
Improved Brain Expression of Anti-Amyloid β scFv by Complexation of mRNA Including a Secretion Sequence with PEG-based Block Catiomer.Curr Alzheimer Res. 2017;14(3):295-302. doi: 10.2174/1567205013666161108110031. Curr Alzheimer Res. 2017. PMID: 27829339
-
Intracranial adeno-associated virus-mediated delivery of anti-pan amyloid beta, amyloid beta40, and amyloid beta42 single-chain variable fragments attenuates plaque pathology in amyloid precursor protein mice.J Neurosci. 2006 Nov 15;26(46):11923-8. doi: 10.1523/JNEUROSCI.2795-06.2006. J Neurosci. 2006. PMID: 17108166 Free PMC article.
-
[Transmission of pathogenic protein aggregates in Alzheimer's disease].Mol Biol (Mosk). 2017 May-Jun;51(3):418-422. doi: 10.7868/S0026898417030144. Mol Biol (Mosk). 2017. PMID: 28707657 Review. Russian.
-
APP transgenic modeling of Alzheimer's disease: mechanisms of neurodegeneration and aberrant neurogenesis.Brain Struct Funct. 2010 Mar;214(2-3):111-26. doi: 10.1007/s00429-009-0232-6. Epub 2009 Nov 29. Brain Struct Funct. 2010. PMID: 20091183 Free PMC article. Review.
Cited by
-
TARGETING SOLUBLE AMYLOID-BETA OLIGOMERS WITH A NOVEL NANOBODY.Res Sq [Preprint]. 2024 Mar 15:rs.3.rs-3944211. doi: 10.21203/rs.3.rs-3944211/v1. Res Sq. 2024. Update in: Sci Rep. 2024 Jul 12;14(1):16086. doi: 10.1038/s41598-024-66970-6. PMID: 38559050 Free PMC article. Updated. Preprint.
-
Targeting soluble amyloid-beta oligomers with a novel nanobody.Sci Rep. 2024 Jul 12;14(1):16086. doi: 10.1038/s41598-024-66970-6. Sci Rep. 2024. PMID: 38992064 Free PMC article.
-
Traumatic Brain Injury in Mice Generates Early-Stage Alzheimer's Disease Related Protein Pathology that Correlates with Neurobehavioral Deficits.Mol Neurobiol. 2024 Oct;61(10):7567-7582. doi: 10.1007/s12035-024-04035-5. Epub 2024 Feb 27. Mol Neurobiol. 2024. PMID: 38411868 Free PMC article.
-
Bioactive Compounds and Their Derivatives: An Insight into Prospective Phytotherapeutic Approach against Alzheimer's Disease.Oxid Med Cell Longev. 2022 Apr 11;2022:5100904. doi: 10.1155/2022/5100904. eCollection 2022. Oxid Med Cell Longev. 2022. PMID: 35450410 Free PMC article. Review.
-
Generation of nanobodies with conformational specificity for tau oligomers that recognize tau aggregates from human Alzheimer's disease samples.Biomater Sci. 2024 Nov 19;12(23):6033-6046. doi: 10.1039/d4bm00707g. Biomater Sci. 2024. PMID: 39434503 Free PMC article.
References
-
- Hamley I.W. The amyloid beta peptide: A chemist's perspective. Role in Alzheimer's and fibrillization. Chem. Rev. 2012;112:5147–5192. - PubMed
-
- Roher A.E., Chaney M.O., Kuo Y.M., Webster S.D., Stine W.B., Haverkamp L.J., Woods A.S., Cotter R.J., Tuohy J.M., Krafft G.A., Bonnell B.S., Emmerling M.R. Morphology and toxicity of Abeta-(1-42) dimer derived from neuritic and vascular amyloid deposits of Alzheimer's disease. J. Biol. Chem. 1996;271:20631–20635. - PubMed
-
- Haass C., Selkoe D.J. Soluble protein oligomers in neurodegeneration: Lessons from the Alzheimer's amyloid beta-peptide. Nat. Rev. Mol. Cell Biol. 2007;8:101–112. - PubMed
-
- Shankar G.M., Li S., Mehta T.H., Garcia-Munoz A., Shepardson N.E., Smith I., Brett F.M., Farrell M.A., Rowan M.J., Lemere C.A., Regan C.M., Walsh D.M., Sabatini B.L., Selkoe D.J. Amyloid-beta protein dimers isolated directly from Alzheimer's brains impair synaptic plasticity and memory. Nat. Med. 2008;14:837–842. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

