Altered Cl- homeostasis hinders forebrain GABAergic interneuron migration in a mouse model of intellectual disability
- PMID: 33376209
- PMCID: PMC7812749
- DOI: 10.1073/pnas.2016034118
Altered Cl- homeostasis hinders forebrain GABAergic interneuron migration in a mouse model of intellectual disability
Abstract
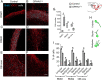
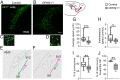
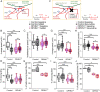
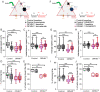
Impairments of inhibitory circuits are at the basis of most, if not all, cognitive deficits. The impact of OPHN1, a gene associate with intellectual disability (ID), on inhibitory neurons remains elusive. We addressed this issue by analyzing the postnatal migration of inhibitory interneurons derived from the subventricular zone in a validated mouse model of ID (OPHN1-/y mice). We found that the speed and directionality of migrating neuroblasts were deeply perturbed in OPHN1-/y mice. The significant reduction in speed was due to altered chloride (Cl-) homeostasis, while the overactivation of the OPHN1 downstream signaling pathway, RhoA kinase (ROCK), caused abnormalities in the directionality of the neuroblast progression in mutants. Blocking the cation-Cl- cotransporter KCC2 almost completely rescued the migration speed while proper directionality was restored upon ROCK inhibition. Our data unveil a strong impact of OPHN1 on GABAergic inhibitory interneurons and identify putative targets for successful therapeutic approaches.
Keywords: 2P imaging; Cl homeostasis; OPHN1; intellectual disability; interneuron migration.
Conflict of interest statement
The authors declare no competing interest.
Figures


Similar articles
-
ROCK/PKA Inhibition Rescues Hippocampal Hyperexcitability and GABAergic Neuron Alterations in a Oligophrenin-1 Knock-Out Mouse Model of X-Linked Intellectual Disability.J Neurosci. 2020 Mar 25;40(13):2776-2788. doi: 10.1523/JNEUROSCI.0462-19.2020. Epub 2020 Feb 25. J Neurosci. 2020. PMID: 32098904 Free PMC article.
-
Inhibition of RhoA pathway rescues the endocytosis defects in Oligophrenin1 mouse model of mental retardation.Hum Mol Genet. 2009 Jul 15;18(14):2575-83. doi: 10.1093/hmg/ddp189. Epub 2009 Apr 28. Hum Mol Genet. 2009. PMID: 19401298 Free PMC article.
-
Pharmacological rescue of adult hippocampal neurogenesis in a mouse model of X-linked intellectual disability.Neurobiol Dis. 2017 Apr;100:75-86. doi: 10.1016/j.nbd.2017.01.003. Epub 2017 Jan 12. Neurobiol Dis. 2017. PMID: 28088401 Free PMC article.
-
Expanding the phenotypic spectrum associated with OPHN1 mutations: Report of 17 individuals with intellectual disability but no cerebellar hypoplasia.Eur J Med Genet. 2018 Aug;61(8):442-450. doi: 10.1016/j.ejmg.2018.03.002. Epub 2018 Mar 3. Eur J Med Genet. 2018. PMID: 29510240 Review.
-
Rho GTPase signaling at the synapse: implications for intellectual disability.Exp Cell Res. 2013 Sep 10;319(15):2368-74. doi: 10.1016/j.yexcr.2013.05.033. Epub 2013 Jun 14. Exp Cell Res. 2013. PMID: 23769912 Review.
Cited by
-
Network instability dynamics drive a transient bursting period in the developing hippocampus in vivo.Elife. 2022 Dec 19;11:e82756. doi: 10.7554/eLife.82756. Elife. 2022. PMID: 36534089 Free PMC article.
-
Daily rhythm in cortical chloride homeostasis underpins functional changes in visual cortex excitability.Nat Commun. 2023 Nov 4;14(1):7108. doi: 10.1038/s41467-023-42711-7. Nat Commun. 2023. PMID: 37925453 Free PMC article.
-
How Staying Negative Is Good for the (Adult) Brain: Maintaining Chloride Homeostasis and the GABA-Shift in Neurological Disorders.Front Mol Neurosci. 2022 Jul 8;15:893111. doi: 10.3389/fnmol.2022.893111. eCollection 2022. Front Mol Neurosci. 2022. PMID: 35875665 Free PMC article. Review.
-
Potential cardiotoxicity induced by Euodiae Fructus: In vivo and in vitro experiments and untargeted metabolomics research.Front Pharmacol. 2022 Oct 24;13:1028046. doi: 10.3389/fphar.2022.1028046. eCollection 2022. Front Pharmacol. 2022. PMID: 36353487 Free PMC article.
-
Optogenetic Methods to Investigate Brain Alterations in Preclinical Models.Cells. 2022 Jun 5;11(11):1848. doi: 10.3390/cells11111848. Cells. 2022. PMID: 35681542 Free PMC article. Review.
References
-
- Billuart P., et al. , Oligophrenin 1 encodes a rho-GAP protein involved in X-linked mental retardation. Pathol. Biol. (Paris) 46, 678 (1998). - PubMed
-
- Fauchereau F., et al. , The RhoGAP activity of OPHN1, a new F-actin-binding protein, is negatively controlled by its amino-terminal domain. Mol. Cell. Neurosci. 23, 574–586 (2003). - PubMed
-
- Govek E. E., et al. , The X-linked mental retardation protein oligophrenin-1 is required for dendritic spine morphogenesis. Nat. Neurosci. 7, 364–372 (2004). - PubMed
-
- Billuart P., Chelly J., Gilgenkrantz S., [X-linked mental retardation]. Med. Sci. (Paris) 21, 947–953 (2005). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

