Uncovering the chemistry behind inducible morphological defences in the crustacean Daphnia magna via micro-Raman spectroscopy
- PMID: 33376239
- PMCID: PMC7772340
- DOI: 10.1038/s41598-020-79755-4
Uncovering the chemistry behind inducible morphological defences in the crustacean Daphnia magna via micro-Raman spectroscopy
Abstract
The widespread distribution of Crustacea across every aquatic ecological niche on Earth is enabled due to their exoskeleton's versatile properties. Especially mineralization of the exoskeleton provides protection against diverse environmental threats. Thereby, the exoskeleton of some entomostracans is extremely phenotypically plastic, especially in response to predators. For instance, the freshwater zooplankton Daphnia forms conspicuous inducible morphological defenses, such as helmets, and can increase the stability of its exoskeleton, which renders them less vulnerable to predation. In this study, we reveal for the first time the chemical composition of the exoskeleton of Daphnia magna, using Raman spectroscopy, to be composed of α-chitin and proteins with embedded amorphous calcium carbonate (ACC). Furthermore, we reveal the exoskeleton's chemical changes associated with inducible defense mechanisms in the form of more substantial mineralization, which is probably correlated with enhanced carapace stability. We, therefore, highlight the importance of calcium-biominerals for inducible morphological defenses in Daphnia.
Conflict of interest statement
The authors declare no competing interests.
Figures



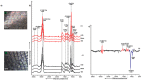
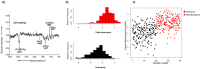
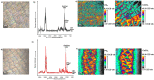
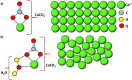
References
-
- Boßelmann F, Romano P, Fabritius H, Raabe D, Epple M. The composition of the exoskeleton of two crustacea: The American lobster Homarusamericanus and the edible crab Cancerpagurus. Thermochim. Acta. 2007;463:65–68. doi: 10.1016/j.tca.2007.07.018. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

