Clopidogrel-Induced Gastric Injury in Rats is Attenuated by Stable Gastric Pentadecapeptide BPC 157
- PMID: 33376304
- PMCID: PMC7763470
- DOI: 10.2147/DDDT.S284163
Clopidogrel-Induced Gastric Injury in Rats is Attenuated by Stable Gastric Pentadecapeptide BPC 157
Abstract
Aim: Although Clopidogrel is safe in healthy volunteers, it can induce recurrence of gastric ulcers in high-risk patients. Here, we investigated the protective effect of the natural product, stable gastric pentadecapeptide 157 (BPC 157) on Clopidogrel-induced gastric injury.
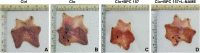
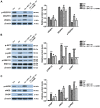
Methods: We used acetic acid to induce gastric ulcer in Sprague Dawley rats. Clopidogrel alone or in combination with BPC 157 or L-NAME (nitric oxide system blockade) were administered after healing of acetic acid-induced ulcer. One percent methylcellulose solution was used as control. Ulcer recurrence rate and the ulcer index were compared between these groups. Gastric mucosal apoptosis rate, microscopic inflammation activity and angiogenesis markers vascular endothelial growth factor A (VEGF-A) and CD34 were examined by TUNEL, histological evaluations (HE) and immunohistochemistry (IHC). Pathways involved, expressions of endoplasmic reticulum (ER) stress apoptosis marker CHOP, angiogenic markers VEGF-A and its receptor VEGFR1, and endothelial NO synthase (eNOS) were all analyzed by Western blot.
Results: This study indicated that Clopidogrel significantly induced the gastric ulcers recurrence, severe inflammation and ER stress related apoptosis of the gastric mucosa, suppressed the synthesis of angiogenic markers and eNOS. Furthermore, Clopidrogel intervention resulted in the activation of protein kinase B (AKT) and p38 mitogen-activated protein kinase (p38/MAPK). BPC 157 attenuated the gastric mucosal damage caused by Clopidogrel and reversed these molecular effects. However, NO blockade L-NAME weakened the protective effect and thus the molecular effects of BPC 157 on gastric mucosa.
Conclusion: In conclusion, these results suggest that BPC 157 inhibited Clopidogrel-induced gastric mucosa injury partially by inhibition of gastric mucosa cell ER stress-mediated apoptosis and inflammation, and promoting gastric mucosa angiogenesis via VEGF-A/VEGFR1 mediated-AKT/p38/MAPK signaling pathways.
Keywords: AKT; BPC 157; angiogenesis; clopidogrel; p38/MAPK.
© 2020 Wu et al.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures



Similar articles
-
Clopidogrel inhibits angiogenesis of gastric ulcer healing via downregulation of vascular endothelial growth factor receptor 2.J Formos Med Assoc. 2016 Sep;115(9):764-72. doi: 10.1016/j.jfma.2015.07.022. Epub 2015 Aug 24. J Formos Med Assoc. 2016. PMID: 26315480
-
Protective effects of pentadecapeptide BPC 157 on gastric ulcer in rats.World J Gastroenterol. 2004 Apr 1;10(7):1032-6. doi: 10.3748/wjg.v10.i7.1032. World J Gastroenterol. 2004. PMID: 15052688 Free PMC article.
-
Celecoxib-induced gastrointestinal, liver and brain lesions in rats, counteraction by BPC 157 or L-arginine, aggravation by L-NAME.World J Gastroenterol. 2017 Aug 7;23(29):5304-5312. doi: 10.3748/wjg.v23.i29.5304. World J Gastroenterol. 2017. PMID: 28839430 Free PMC article.
-
Stable gastric pentadecapeptide BPC 157 in trials for inflammatory bowel disease (PL-10, PLD-116, PL 14736, Pliva, Croatia). Full and distended stomach, and vascular response.Inflammopharmacology. 2006 Dec;14(5-6):214-21. doi: 10.1007/s10787-006-1531-7. Inflammopharmacology. 2006. PMID: 17186181 Review.
-
Stable gastric pentadecapeptide BPC 157-NO-system relation.Curr Pharm Des. 2014;20(7):1126-35. doi: 10.2174/13816128113190990411. Curr Pharm Des. 2014. PMID: 23755725 Review.
Cited by
-
New studies with stable gastric pentadecapeptide protecting gastrointestinal tract. significance of counteraction of vascular and multiorgan failure of occlusion/occlusion-like syndrome in cytoprotection/organoprotection.Inflammopharmacology. 2024 Oct;32(5):3119-3161. doi: 10.1007/s10787-024-01499-8. Epub 2024 Jul 9. Inflammopharmacology. 2024. PMID: 38980576 Review.
-
A comprehensive update on the potential of curcumin to enhance chemosensitivity in colorectal cancer.Pharmacol Rep. 2025 Feb;77(1):103-123. doi: 10.1007/s43440-024-00652-y. Epub 2024 Sep 20. Pharmacol Rep. 2025. PMID: 39304638 Review.
-
Stable Gastric Pentadecapeptide BPC 157 Therapy: Effect on Reperfusion Following Maintained Intra-Abdominal Hypertension (Grade III and IV) in Rats.Pharmaceuticals (Basel). 2023 Nov 2;16(11):1554. doi: 10.3390/ph16111554. Pharmaceuticals (Basel). 2023. PMID: 38004420 Free PMC article.
-
Influence of antiplatelet drugs on gastric ulcer healing after endoscopic submucosal dissection in patients with early gastric cancer.DEN Open. 2025 Feb 11;5(1):e70070. doi: 10.1002/deo2.70070. eCollection 2025 Apr. DEN Open. 2025. PMID: 39935747 Free PMC article.
-
Cytoprotective gastric pentadecapeptide BPC 157 resolves major vessel occlusion disturbances, ischemia-reperfusion injury following Pringle maneuver, and Budd-Chiari syndrome.World J Gastroenterol. 2022 Jan 7;28(1):23-46. doi: 10.3748/wjg.v28.i1.23. World J Gastroenterol. 2022. PMID: 35125818 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

