Methotrexate (MTX) Plus Hydroxychloroquine versus MTX Plus Leflunomide in Patients with MTX-Resistant Active Rheumatoid Arthritis: A 2-Year Cohort Study in Real World
- PMID: 33376379
- PMCID: PMC7755368
- DOI: 10.2147/JIR.S282249
Methotrexate (MTX) Plus Hydroxychloroquine versus MTX Plus Leflunomide in Patients with MTX-Resistant Active Rheumatoid Arthritis: A 2-Year Cohort Study in Real World
Abstract
Purpose: To compare the efficacy, safety, and cost-effectiveness of methotrexate (MTX) plus hydroxychloroquine (HCQ) vs MTX plus leflunomide (LEF) in established rheumatoid arthritis (RA) with inadequate response to MTX monotherapy in a real-world Chinese cohort.
Patients and methods: A prospective RA cohort (n=549) was screened with eligible patients who had inadequate response (disease activity score in 28 joints using erythrocyte sedimentation rate, DAS28-ESR>3.2) to initial MTX monotherapy and subsequently received either MTX+HCQ or MTX+LEF. Propensity score matching (PSM) was applied to adjust the possible baseline confounders between two groups. The primary outcome was the proportion of patients achieving first remission (DAS28-ESR<2.6) during follow-up by log rank test. Secondary outcomes were changes of DAS28, glucocorticoids (GCs) exposure, safety, cost-effectiveness, sustained remission, and low disease activity (LDA) rate after 24-month follow-up.
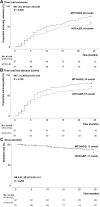
Results: Overall, 222 eligible patients were subjected to the aforementioned two treatment protocols (MTX+HCQ, n=102; MTX+LEF, n=120). After PSM adjustment, 97 patients in each group were analyzed. A higher remission rate was observed in the MTX+HCQ group than in the MTX+LEF group (70.1% vs 56.7%, P=0.048). The median time to remission was 11 and 16 months in the two groups, respectively. At the endpoint, more patients achieved remission (46.8% vs 32.5%, P=0.063) and maintained sustained LDA in the HCQ group (53.2% vs 38.6%, P=0.062) and also more patients withdrew GCs in this group (32% vs 16.7%, P=0.053) than those in the LEF group. Safety profiles were non-alarming, with no significant difference between the two groups. The incremental cost-effectiveness ratio yielded by MTX+HCQ over MTX+LEF was $1,111.8 per quality-adjusted life-year (QALY), within the cost-effective threshold set as the per capita gross domestic product (GDP) of China.
Conclusion: The MTX+HCQ combination was seemingly superior to MTX+LEF in a real-world cohort of Chinese RA patients with inadequate response to methotrexate monotherapy in respect of the efficacy and cost-effectiveness.
Keywords: cost-effectiveness; efficacy; hydroxychloroquine; leflunomide; methotrexate-resistant; rheumatoid arthritis.
© 2020 Zhang et al.
Conflict of interest statement
The authors declare that they have no conflict of interests.
Figures
Similar articles
-
Effectiveness of maintenance therapy with methotrexate compared with leflunomide for patients with RA having achieved disease control with both these drugs: results of a predefined sub-analysis of CareRA, a pragmatic RCT.Clin Rheumatol. 2020 Sep;39(9):2593-2601. doi: 10.1007/s10067-020-05008-4. Epub 2020 Mar 12. Clin Rheumatol. 2020. PMID: 32166429 Clinical Trial.
-
A randomized comparative effectiveness study of oral triple therapy versus etanercept plus methotrexate in early aggressive rheumatoid arthritis: the treatment of Early Aggressive Rheumatoid Arthritis Trial.Arthritis Rheum. 2012 Sep;64(9):2824-35. doi: 10.1002/art.34498. Arthritis Rheum. 2012. PMID: 22508468 Free PMC article. Clinical Trial.
-
Short-term efficacy of etanercept plus methotrexate vs combinations of disease-modifying anti-rheumatic drugs with methotrexate in established rheumatoid arthritis.Rheumatology (Oxford). 2014 Nov;53(11):1984-93. doi: 10.1093/rheumatology/keu235. Epub 2014 Jun 6. Rheumatology (Oxford). 2014. PMID: 24907147 Clinical Trial.
-
Effectiveness and Safety of Iguratimod Monotherapy or Combined With Methotrexate in Treating Rheumatoid Arthritis: A Systematic Review and Meta-Analysis.Front Pharmacol. 2022 Aug 5;13:911810. doi: 10.3389/fphar.2022.911810. eCollection 2022. Front Pharmacol. 2022. PMID: 35991879 Free PMC article.
-
Sarilumab for Previously-Treated Moderate or Severe Rheumatoid Arthritis: An Evidence Review Group Perspective of a NICE Single Technology Appraisal.Pharmacoeconomics. 2018 Dec;36(12):1427-1437. doi: 10.1007/s40273-018-0677-7. Pharmacoeconomics. 2018. PMID: 29882210 Review.
Cited by
-
Network Pharmacology-Based Strategy Integrated with Molecular Docking and In Vitro Experimental Validation to Explore the Underlying Mechanism of Fangji Huangqi Decoction in Treating Rheumatoid Arthritis.ACS Omega. 2024 Jul 10;9(29):31878-31889. doi: 10.1021/acsomega.4c03495. eCollection 2024 Jul 23. ACS Omega. 2024. PMID: 39072058 Free PMC article.
-
The effective threshold dose of etanercept in patients with methotrexate-resistant rheumatoid arthritis.Clin Rheumatol. 2023 Oct;42(10):2777-2786. doi: 10.1007/s10067-023-06659-9. Epub 2023 Jul 7. Clin Rheumatol. 2023. PMID: 37415053 Free PMC article.
-
The Role of Hydroxychloroquine in the Management of Rheumatic Disorders: A Comprehensive Review.Basic Clin Pharmacol Toxicol. 2025 Aug;137(2):e70082. doi: 10.1111/bcpt.70082. Basic Clin Pharmacol Toxicol. 2025. PMID: 40693372 Free PMC article. Review.
-
Traditional Chinese Medicine Qingre Huoxue Treatment vs. the Combination of Methotrexate and Hydroxychloroquine for Active Rheumatoid Arthritis: A Multicenter, Double-Blind, Randomized Controlled Trial.Front Pharmacol. 2021 May 25;12:679588. doi: 10.3389/fphar.2021.679588. eCollection 2021. Front Pharmacol. 2021. PMID: 34113254 Free PMC article.
-
Where Do We Stand in the Management of Rheumatoid Arthritis Ahead of EULAR/ACR 2025?Clin Pract. 2025 May 28;15(6):103. doi: 10.3390/clinpract15060103. Clin Pract. 2025. PMID: 40558221 Free PMC article. Review.
References
-
- van Vollenhoven RF, Ernestam S, Geborek P, et al. Addition of infliximab compared with addition of sulfasalazine and hydroxychloroquine to methotrexate in patients with early rheumatoid arthritis (Swefot trial): 1-year results of a randomised trial. Lancet. 2009;374(9688):459–466. doi:10.1016/s0140-6736(09)60944-2 - DOI - PubMed
-
- Moreland LW, O’Dell JR, Paulus HE, et al. A randomized comparative effectiveness study of oral triple therapy versus etanercept plus methotrexate in early aggressive rheumatoid arthritis: the treatment of early aggressive rheumatoid arthritis trial. Arthritis Rheum. 2012;64(9):2824–2835. doi:10.1002/art.34498 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous