In-vitro Evaluations of Quality Control Parameters of Paracetamol Tablets Marketed in Gondar City, Northwest Ethiopia
- PMID: 33376411
- PMCID: PMC7762764
- DOI: 10.2147/DHPS.S282420
In-vitro Evaluations of Quality Control Parameters of Paracetamol Tablets Marketed in Gondar City, Northwest Ethiopia
Abstract
Background: The aim of this research was to evaluate quality control parameters of available brands of paracetamol tablets in Gondar city since standard quality parameters are essential for a better quality of the product. The different brands of paracetamol tablets were obtained from local pharmacies in Gondar town and the University of Gondar (UOG) hospital pharmacies.
Methods: Five brands of paracetamol, from each, 102 tablets were collected from private pharmacies, government health centers, and UOG pharmacies. The popular brands in the city, Panadol, Para-denk, Paramol, Paracetamol (EPHARM), and Cadimol, conventional tablets of 500 mg strength were chosen and the tablets were assessed for different quality parameters: weight variation, hardness, friability, disintegration, dissolution, and drug content (assay) using compendial methods. The tablets were evaluated to check if they comply with the specifications of USP (United States Pharmacopeia).
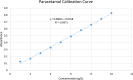
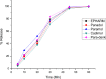
Results: From the results, it was observed that all the brands of paracetamol have passed the tests and met the specifications of USP. Results of weight variation, hardness, friability, and disintegration time ranged from 0.46 to 1.11%, 117.0 to 174.70 N, 0.07 to 0.63%, and 01 to 08 minutes for all the tablets, respectively. The dissolution profiles of all the brands are within the acceptable label claim. The assay results showed that the drug content of the paracetamol brands ranged from 95.04% to 106.81%. The dissolution rate was significantly different (p < 0.05) as compared to code 1 with all brands tested at 30 minutes. The disintegration time of different brands was also significantly different from the comparator (code 1) except code 2.
Conclusion: Based on the finding from this study, there were no significant deviations from pharmacopeia standards and specifications. The brands studied were safe enough and could be used to achieve the desired therapeutic effect.
Keywords: Ethiopia; Gondar; paracetamol; quality control.
© 2020 Abebe et al.
Conflict of interest statement
The authors report no conflicts of interest for this work.
Figures
Similar articles
-
In vitro Comparative Quality Assessment of Different Brands of Furosemide Tablets Marketed in Northwest Ethiopia.Drug Des Devel Ther. 2020 Nov 24;14:5119-5128. doi: 10.2147/DDDT.S280203. eCollection 2020. Drug Des Devel Ther. 2020. PMID: 33262573 Free PMC article.
-
In vitro comparative quality evaluation of different brands of carbamazepine tablets commercially available in Dessie town, Northeast Ethiopia.BMC Pharmacol Toxicol. 2023 May 25;24(1):35. doi: 10.1186/s40360-023-00670-1. BMC Pharmacol Toxicol. 2023. PMID: 37231520 Free PMC article.
-
Evaluation of Seven Different Brands of Metformin Hydrochloride Tablets Available in the Market in Gondar City, Ethiopia.Drug Healthc Patient Saf. 2024 Feb 1;16:19-28. doi: 10.2147/DHPS.S419522. eCollection 2024. Drug Healthc Patient Saf. 2024. PMID: 38318121 Free PMC article.
-
Comparative Quality Control Study of Different Brands of Diclofenac Sodium Tablet Available in Local and Government Pharmacies by In-Vitro Testing.Cureus. 2020 Nov 5;12(11):e11348. doi: 10.7759/cureus.11348. Cureus. 2020. PMID: 33304683 Free PMC article.
-
In vitro comparative quality assessment of different brands of norfloxacin tablets available in Jimma, Southwest Ethiopia.Drug Des Devel Ther. 2019 Apr 17;13:1241-1249. doi: 10.2147/DDDT.S189524. eCollection 2019. Drug Des Devel Ther. 2019. PMID: 31118565 Free PMC article.
Cited by
-
Cost-effective track and trace technology for poor-quality chemotherapeutic pharmaceuticals in resource-limited countries: a review of the Chemotherapeutic Paper Analytical Device.Front Med Technol. 2024 Nov 21;6:1436614. doi: 10.3389/fmedt.2024.1436614. eCollection 2024. Front Med Technol. 2024. PMID: 39640069 Free PMC article. Review.
-
Quality of veterinary anthelmintic drugs marketed in Gondar Zones, North West Ethiopia; presence of poor-quality medicines.Heliyon. 2023 Jul 6;9(7):e18023. doi: 10.1016/j.heliyon.2023.e18023. eCollection 2023 Jul. Heliyon. 2023. PMID: 37483800 Free PMC article.
-
Post-marketing quality surveillance of selected antibacterial agents marketed in porous borders; the case of Ethiopia-Sudan-Eritrea border.PLoS One. 2024 Aug 12;19(8):e0308223. doi: 10.1371/journal.pone.0308223. eCollection 2024. PLoS One. 2024. PMID: 39133679 Free PMC article.
-
Formulation, Quality Control and Stability Study of Pediatric Oral Dextrose Gel.Pharmaceuticals (Basel). 2025 Feb 3;18(2):204. doi: 10.3390/ph18020204. Pharmaceuticals (Basel). 2025. PMID: 40006018 Free PMC article.
References
-
- Teklu L, Adugna E, Ashenef A. Quality evaluation of paracetamol tablets obtained from the common shops (kiosks) in Addis Ababa, Ethiopia. Int J Pharm Sci Res. 2014;5(8):3502.
-
- Mosharraf Z Determination of the Quality Control Parameters of Paracetamol Tablets in Bangladesh Pharma Market. 2012.
-
- Marsden A, Shahtout A. International organization for standardization. Clin Lab Manag. 2013;447–450.
LinkOut - more resources
Full Text Sources
Miscellaneous