TIR-Domain-Containing Adaptor-Inducing Interferon- β (TRIF) Is Involved in Glucose Metabolism in Adipose Tissue through the Insulin/AKT Signaling Pathway
- PMID: 33376487
- PMCID: PMC7744180
- DOI: 10.1155/2020/6942307
TIR-Domain-Containing Adaptor-Inducing Interferon- β (TRIF) Is Involved in Glucose Metabolism in Adipose Tissue through the Insulin/AKT Signaling Pathway
Abstract
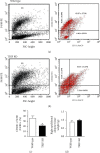
Obesity significantly increases the risk of developing type 2 diabetes mellitus and other metabolic diseases. Obesity is associated with chronic low-grade inflammation in white adipose tissues, which is thought to play an essential role in developing insulin resistance. Many lines of evidence indicate that toll-like receptors (TLRs) and their downstream signaling pathways are involved in development of chronic low-grade inflammation and insulin resistance, which are associated with obesity. Mice lacking molecules positively involved in the TLR signaling pathways are generally protected from high-fat diet-induced inflammation and insulin resistance. In this study, we have determined the effects of genetic deficiency of toll/interleukin-1 receptor-domain-containing adaptor-inducing interferon-β (TRIF) on food intake, bodyweight, glucose metabolism, adipose tissue macrophage polarization, and insulin signaling in normal chow diet-fed mice to investigate the role of the TRIF-dependent TLR signaling in adipose tissue metabolism and inflammation. TRIF deficiency (TRIF-/-) increased food intake and bodyweight. The significant increase in bodyweight in TRIF-/- mice was discernible as early as 24 weeks of age and sustained thereafter. TRIF-/- mice showed impaired glucose tolerance in glucose tolerance tests, but their insulin tolerance tests were similar to those in TRIF+/+ mice. Although no difference was found in the epididymal adipose mass between the two groups, the percentage of CD206+ M2 macrophages in epididymal adipose tissue decreased in TRIF-/- mice compared with those in TRIF+/+ mice. Furthermore, activation of epididymal adipose AKT in response to insulin stimulation was remarkably diminished in TRIF-/- mice compared with TRIF+/+ mice. Our results indicate that the TRIF-dependent TLR signaling contributes to maintaining insulin/AKT signaling and M2 macrophages in epididymal adipose tissue under a normal chow diet and provide new evidence that TLR4-targeted therapies for type 2 diabetes require caution.
Copyright © 2020 Junling Yang and Ken-Ichiro Fukuchi.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures



References
-
- Adeva-Andany M. M., Fernandez-Fernandez C., Carneiro-Freire N., Castro-Quintela E., Pedre-Pineiro A., Seco-Filgueira M. Insulin resistance underlies the elevated cardiovascular risk associated with kidney disease and glomerular hyperfiltration. Reviews in Cardiovascular Medicine. 2020;21(1):41–56. doi: 10.31083/j.rcm.2020.01.5102. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

