Severe SARS-CoV-2 patients develop a higher specific T-cell response
- PMID: 33376594
- PMCID: PMC7757425
- DOI: 10.1002/cti2.1217
Severe SARS-CoV-2 patients develop a higher specific T-cell response
Abstract
Objectives: Assessment of the adaptive immune response against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is crucial for studying long-term immunity and vaccine strategies. We quantified IFNγ-secreting T cells reactive against the main viral SARS-CoV-2 antigens using a standardised enzyme-linked immunospot assay (ELISpot).
Methods: Overlapping peptide pools built from the sequences of M, N and S viral proteins and a mix (MNS) were used as antigens. Using IFNγ T-CoV-Spot assay, we assessed T-cell and antibody responses in mild, moderate and severe SARS-CoV-2 patients and in control samples collected before the outbreak.
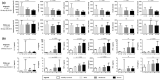
Results: Specific T cells were assessed in 60 consecutive patients (mild, n = 26; moderate, n = 10; and severe patients, n = 24) during their follow-up (median time from symptom onset [interquartile range]: 36 days [28;53]). T cells against M, N and S peptide pools were detected in n = 60 (100%), n = 56 (93.3%), n = 55 patients (91.7%), respectively. Using the MNS mix, IFNγ T-CoV-Spot assay showed a specificity of 96.7% (95% CI, 88.5-99.6%) and a specificity of 90.3% (75.2-98.0%). The frequency of reactive T cells observed with M, S and MNS mix pools correlated with severity and with levels of anti-S1 and anti-RBD serum antibodies.
Conclusion: IFNγ T-CoV-Spot assay is a reliable method to explore specific T cells in large cohorts of patients. This test may become a useful tool to assess the long-lived memory T-cell response after vaccination. Our study demonstrates that SARS-CoV-2 patients developing a severe disease achieve a higher adaptive immune response.
Keywords: ELISpot; SARS‐CoV‐2; T cells.
© 2020 The Authors. Clinical & Translational Immunology published by John Wiley & Sons Australia, Ltd on behalf of Australian and New Zealand Society for Immunology, Inc.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


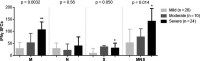



References
LinkOut - more resources
Full Text Sources
Miscellaneous
