Co-Cr-Mo-Cu alloys for clinical implants with osteogenic effect by increasing bone induction, formation and development in a rabbit model
- PMID: 33376752
- PMCID: PMC7750714
- DOI: 10.1093/burnst/tkaa036
Co-Cr-Mo-Cu alloys for clinical implants with osteogenic effect by increasing bone induction, formation and development in a rabbit model
Abstract
Background: Co-Cr-Mo alloy has been widely used in clinical implants because of its excellent mechanical and anti-corrosion properties, but there is an urgent need to address its disadvantages, such as implant-related infections and implant loosening. We synthesized Co-Cr-Mo-Cu (Co-Cu) alloys with different Cu contents to modify implant performance to be suitable as a bone-compatible implant material.
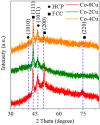
Methods: Microstructure, phase content and mechanical properties of the Co-Cr-Mo alloy were characterized. Histological and immunohistochemical analyses were performed after implantation in rabbits. The experimental alloy was implanted on the lateral side of the lower tibial condyle and the tibial nodule.
Results: Phase content and mechanical properties revealed that the crystallographic structure and wear resistance were changed. Experimental implantation results demonstrated that osteogenic capability was markedly enhanced, ascribed to the excellent antibacterial and osseointegration capacities of Cu phases, and with the release of Cu ions. In particular, Co-Cu alloy containing 2 wt% Cu exhibited the best osteogenic performance among all samples.
Conclusions: The results indicated that osteogenic performance of the Co-Cr-Mo alloy could be enhanced by adding Cu. In particular, the Co-2Cu alloy exhibited the best properties according to both immunohistochemical and histological analyses. Our study not only provides deep insight into the osteogenic effect of Cu but presents a new Co-Cu alloy for clinical implants.
Keywords: Clinical application; Co-Cr-Mu-Cu alloy; Cu ion; Implantable operation; Osteogenic capability.
© The Author(s) 2020. Published by Oxford University Press.
Figures












References
-
- Hodgson AWE, Kurz S, Virtanen S, Fervel V, Olsson COA, Mischler S. Passive and transpassive behaviour of CoCrMo in simulated biological solutions. Electrochim Acta. 2014;49:2167–78.
-
- Xiang DD, Wang P, Tan XP, Chandra S, Wang C, Nai MLS, et al. Anisotropic microstructure and mechanical properties of additively manufactured Co–Cr–Mo alloy using selective electron beam melting for orthopedic implants. Mater Sci Eng A Struct Mater. 2019;765:138270 10.1016/j.msea.2019.138270. - DOI
-
- Igual Muñoz A, Casabán Julián L. Influence of electrochemical potential on the tibocorrosion behaviour of high carbon CoCrMo biomedical alloy in simulated body fluids by electrochemical impedance spectroscopy. Electrochim Acta. 2010;55:5428–39.
-
- Wei D, Koizumi Y, Takashima T, Nagasako M, Chiba A. Fatigue improvement of electron beam melting-fabricated biomedical co–Cr–Mo alloy by accessible heat treatment. Mater Res Lett. 2018;6:93–9.
-
- Shah F, Omar O, Suska F, Snis A, Matic A, Emanuelsson L, et al. Long-term osseointegration of 3D printed CoCr constructs with an interconnected open-pore architecture prepared by electron beam melting. Acta Biomater. 2016;36:296–309. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

