Beyond Low-Earth Orbit: Characterizing Immune and microRNA Differentials following Simulated Deep Spaceflight Conditions in Mice
- PMID: 33376970
- PMCID: PMC7756144
- DOI: 10.1016/j.isci.2020.101747
Beyond Low-Earth Orbit: Characterizing Immune and microRNA Differentials following Simulated Deep Spaceflight Conditions in Mice
Abstract
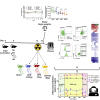
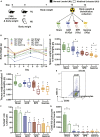
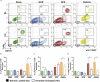
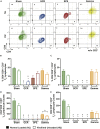
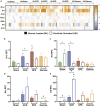
Spaceflight missions can cause immune system dysfunction in astronauts with little understanding of immune outcomes in deep space. This study assessed immune responses in mice following ground-based, simulated deep spaceflight conditions, compared with data from astronauts on International Space Station missions. For ground studies, we simulated microgravity using the hindlimb unloaded mouse model alone or in combination with acute simulated galactic cosmic rays or solar particle events irradiation. Immune profiling results revealed unique immune diversity following each experimental condition, suggesting each stressor results in distinct circulating immune responses, with clear consequences for deep spaceflight. Circulating plasma microRNA sequence analysis revealed involvement in immune system dysregulation. Furthermore, a large astronaut cohort showed elevated inflammation during low-Earth orbit missions, thereby supporting our simulated ground experiments in mice. Herein, circulating immune biomarkers are defined by distinct deep space irradiation types coupled to simulated microgravity and could be targets for future space health initiatives.
Keywords: Immunology; Space Sciences.
© 2020 The Author(s).
Conflict of interest statement
All authors declare no competing interests.
Figures




References
-
- Bagley J., Tian C., Sachs D.H., Iacomini J. T cells mediate resistance to genetically modified bone marrow in lethally irradiated recipients. Transplantation. 2002;74:1454–1460. - PubMed
LinkOut - more resources
Full Text Sources

