The adult human subventricular zone: partial ependymal coverage and proliferative capacity of cerebrospinal fluid
- PMID: 33376983
- PMCID: PMC7750937
- DOI: 10.1093/braincomms/fcaa150
The adult human subventricular zone: partial ependymal coverage and proliferative capacity of cerebrospinal fluid
Abstract
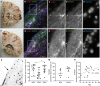
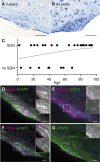
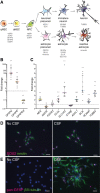
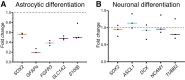
Neurogenesis continues throughout adulthood in specialized regions of the brain. One of these regions is the subventricular zone. During brain development, neurogenesis is regulated by a complex interplay of intrinsic and extrinsic cues that control stem-cell survival, renewal and cell lineage specification. Cerebrospinal fluid (CSF) is an integral part of the neurogenic niche in development as it is in direct contact with radial glial cells, and it is important in regulating proliferation and migration. Yet, the effect of CSF on neural stem cells in the subventricular zone of the adult human brain is unknown. We hypothesized a persistent stimulating effect of ventricular CSF on neural stem cells in adulthood, based on the literature, describing bulging accumulations of subventricular cells where CSF is in direct contact with the subventricular zone. Here, we show by immunohistochemistry on post-mortem adult human subventricular zone sections that neural stem cells are in close contact with CSF via protrusions through both intact and incomplete ependymal layers. We are the first to systematically quantify subventricular glial nodules denuded of ependyma and consisting of proliferating neural stem and progenitor cells, and showed that they are present from foetal age until adulthood. Neurosphere, cell motility and differentiation assays as well as analyses of RNA expression were used to assess the effects of CSF of adult humans on primary neural stem cells and a human immortalized neural stem cell line. We show that human ventricular CSF increases proliferation and decreases motility of neural stem cells. Our results also indicate that adult CSF pushes neural stem cells from a relative quiescent to a more active state and promotes neuronal over astrocytic lineage differentiation. Thus, CSF continues to stimulate neural stem cells throughout aging.
Keywords: cerebrospinal fluid; glial nodules; human; neural stem cells; subventricular zone.
© The Author(s) (2020). Published by Oxford University Press on behalf of the Guarantors of Brain.
Figures



References
-
- Adams CW, Abdulla YH, Torres EM, Poston RN. Periventricular lesions in multiple sclerosis: their perivenous origin and relationship to granular ependymitis. Neuropathol Appl Neurobiol 1987; 13: 141–52. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
