Paradoxical lesions, plasticity and active inference
- PMID: 33376985
- PMCID: PMC7750943
- DOI: 10.1093/braincomms/fcaa164
Paradoxical lesions, plasticity and active inference
Abstract
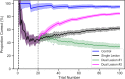
Paradoxical lesions are secondary brain lesions that ameliorate functional deficits caused by the initial insult. This effect has been explained in several ways; particularly by the reduction of functional inhibition, or by increases in the excitatory-to-inhibitory synaptic balance within perilesional tissue. In this article, we simulate how and when a modification of the excitatory-inhibitory balance triggers the reversal of a functional deficit caused by a primary lesion. For this, we introduce in-silico lesions to an active inference model of auditory word repetition. The first in-silico lesion simulated damage to the extrinsic (between regions) connectivity causing a functional deficit that did not fully resolve over 100 trials of a word repetition task. The second lesion was implemented in the intrinsic (within region) connectivity, compromising the model's ability to rebalance excitatory-inhibitory connections during learning. We found that when the second lesion was mild, there was an increase in experience-dependent plasticity that enhanced performance relative to a single lesion. This paradoxical lesion effect disappeared when the second lesion was more severe because plasticity-related changes were disproportionately amplified in the intrinsic connectivity, relative to lesioned extrinsic connections. Finally, this framework was used to predict the physiological correlates of paradoxical lesions. This formal approach provides new insights into the computational and neurophysiological mechanisms that allow some patients to recover after large or multiple lesions.
Keywords: active inference; learning; paradoxical lesions; plasticity; structure–function relationship.
© The Author(s) (2020). Published by Oxford University Press on behalf of the Guarantors of Brain.
Figures



References
-
- Abbott LF, Varela J, Sen K, Nelson S. Synaptic depression and cortical gain control. Science 1997; 275: 221–4. - PubMed
-
- Bestmann S. Computational neurostimulation. Netherlands: Elsevier; 2015.
Grants and funding
LinkOut - more resources
Full Text Sources
