Modulation of Immune Checkpoints by Chemotherapy in Human Colorectal Liver Metastases
- PMID: 33377131
- PMCID: PMC7762777
- DOI: 10.1016/j.xcrm.2020.100160
Modulation of Immune Checkpoints by Chemotherapy in Human Colorectal Liver Metastases
Abstract
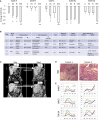
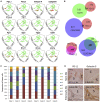
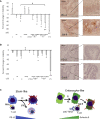
Metastatic colorectal cancer (CRC) is a major cause of cancer-related death, and incidence is rising in younger populations (younger than 50 years). Current chemotherapies can achieve response rates above 50%, but immunotherapies have limited value for patients with microsatellite-stable (MSS) cancers. The present study investigates the impact of chemotherapy on the tumor immune microenvironment. We treat human liver metastases slices with 5-fluorouracil (5-FU) plus either irinotecan or oxaliplatin, then perform single-cell transcriptome analyses. Results from eight cases reveal two cellular subtypes with divergent responses to chemotherapy. Susceptible tumors are characterized by a stemness signature, an activated interferon pathway, and suppression of PD-1 ligands in response to 5-FU+irinotecan. Conversely, immune checkpoint TIM-3 ligands are maintained or upregulated by chemotherapy in CRC with an enterocyte-like signature, and combining chemotherapy with TIM-3 blockade leads to synergistic tumor killing. Our analyses highlight chemomodulation of the immune microenvironment and provide a framework for combined chemo-immunotherapies.
Keywords: PD-L1; TIM3; chemotherapy; colorectal cancer; galectin-9; immune microenvironment; liver metastases; organotypic culture; single-cell analysis; single-cell transcriptome.
© 2020 The Author(s).
Conflict of interest statement
The authors report no competing interests.
Figures




References
-
- Global Burden of Disease Cancer Collaboration. Fitzmaurice C., Allen C., Barber R.M., Barregard L., Bhutta Z.A., Brenner H., Dicker D.J., Chimed-Orchir O., Dandona R. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-years for 32 Cancer Groups, 1990 to 2015: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol. 2017;3:524–548. - PMC - PubMed
-
- Even Storli P., Johnsen G., Juel I.S., Grønbech J.E., Bringeland E.A. Impact of increased resection rates and a liver parenchyma sparing strategy on long-term survival after surgery for colorectal liver metastases. A population-based study. Scand. J. Gastroenterol. 2019;54:890–898. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

