Prognostic impacts of serum uric acid levels in patients with chronic heart failure: insights from the CHART-2 study
- PMID: 33377627
- PMCID: PMC8006606
- DOI: 10.1002/ehf2.12765
Prognostic impacts of serum uric acid levels in patients with chronic heart failure: insights from the CHART-2 study
Abstract
Aims: Prognostic impacts of serum uric acid (UA) levels in patients with chronic heart failure (CHF) remain inconclusive, especially for the whole range of serum UA levels.
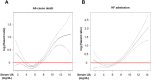
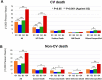
Methods and results: In the Chronic Heart Failure Registry and Analysis in the Tohoku District-2 (CHART-2) study, we enrolled 4652 consecutive patients with CHF and classified them into four groups based on baseline serum UA levels by the Classification and Regression Tree: G1 (<3.8 mg/dL, N = 313), G2 (3.8-7.1 mg/dL, N = 3070), G3 (7.2-9.2 mg/dL, N = 1018), and G4 (>9.2 mg/dL, N = 251). Mean age was 71 ± 12, 69 ± 12, 68 ± 13, and 69 ± 15 years in G1, G2, G3, and G4, respectively (P < 0.001). During the median follow-up of 6.3 years, in G1, G2, G3, and G4, 111 (35%), 905 (29%), 370 (36%), and 139 (55%) patients died and 79 (25%), 729 (24%), 300 (29%), and 115 (46%) experienced heart failure hospitalization, respectively (both P < 0.001). G1 was characterized by a significantly high prevalence of women as compared with G2, G3, and G4 (59%, 32%, 24%, and 23%, respectively). Serum creatinine levels (0.8 ± 0.4, 0.9 ± 0.4, 1.2 ± 0.6, and 1.4 ± 0.8 mg/dL, respectively), prevalence of atrial fibrillation (34%, 39%, 45%, and 50%, respectively), and diuretics use (36%, 45%, 67%, and 89%, respectively) increased from G1, G2, G3 to G4 (all P < 0.001), while left ventricular ejection fraction decreased from G1, G2, G3 to G4 (59 ± 15, 58 ± 15, 54 ± 15, and 52 ± 17%, respectively, P < 0.001). Multivariable Cox proportional hazards models showed that, as compared with G2, both G1 and G4 had increased incidence of all-cause death [adjusted hazard ratio (aHR) 1.34, 95% confidence interval (CI) 1.08-1.67, P = 0.009; aHR 1.28, 95% CI 1.02-1.61, P = 0.037, respectively] and heart failure admission (aHR 1.39, 95% CI 1.09-1.78, P = 0.008 and aHR 1.35, 95% CI, 1.06-1.71, P = 0.014, respectively). This U-shaped relationship was evident in the elderly patients. Furthermore, abnormal transitions to either higher or lower levels of serum UA from G2 were associated with increased mortality (aHR 1.29, 95% CI 1.06-1.57, P = 0.012; aHR 1.57, 95% CI 1.12-2.20, P = 0.009).
Conclusions: These results demonstrate that serum UA levels have the U-shaped prognostic effects and abnormal transitions to either higher or lower levels are associated with poor prognosis in the elderly patients with CHF.
Keywords: Biomarker; Heart failure; Prognosis; Uric acid.
© 2020 The Authors. ESC Heart Failure published by John Wiley & Sons Ltd on behalf of European Society of Cardiology.
Conflict of interest statement
The Department of Evidence‐Based Cardiovascular Medicine, Tohoku University Graduate School of Medicine is supported in part by unrestricted research grants from Daiichi Sankyo (Tokyo, Japan), Bayer Yakuhin (Osaka, Japan), Kyowa Hakko Kirin (Tokyo, Japan), Novartis Pharma (Tokyo, Japan), Dainippon Sumitomo Pharma (Osaka, Japan), Astellas Pharma (Tokyo, Japan), AstraZeneca (Osaka, Japan), Chugai Pharmaceutical (Tokyo, Japan), GlaxoSmithKline (Tokyo, Japan), Kowa Pharmaceutical (Tokyo, Japan), Mitsubishi Tanabe Pharma (Osaka, Japan), Mochida Pharmaceutical (Tokyo, Japan), MSD (Tokyo, Japan), Nippon Boehringer Ingelheim (Tokyo, Japan), Otsuka Pharmaceutical (Tokyo, Japan), Shionogi (Osaka, Japan), and Takeda Pharmaceutical (Osaka, Japan). H. S. has received lecture fees from Bayer Yakuhin (Osaka, Japan) and Daiichi Sankyo (Tokyo, Japan).
Figures




References
-
- Wu AH, Gladden JD, Ahmed M, Ahmed A, Filippatos G. Relation of serum uric acid to cardiovascular disease. Int J Cardiol 2016; 213: 4–7. - PubMed
-
- Cho SK, Chang Y, Kim I, Ryu S. U‐shaped association between serum uric acid level and risk of mortality: a cohort study. Arthritis Rheumatol 2018; 70: 1122–1132. - PubMed
-
- Verdecchia P, Schillaci G, Reboldi G, Santeusanio F, Porcellati C, Brunetti P. Relation between serum uric acid and risk of cardiovascular disease in essential hypertension: the PIUMA study. Hypertension 2000; 36: 1072–1078. - PubMed
-
- Mazza A, Zamboni S, Rizzato E, Pessina AC, Tikhonoff V, Schiavon L, Casiglia E. Serum uric acid shows a J‐shaped trend with coronary mortality in non‐insulin‐dependent diabetic elderly people. The CArdiovascular STudy in the ELderly (CASTEL). Acta Diabetol 2007; 44: 99–105. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

