3D-Printed Multi-Stimuli-Responsive Mobile Micromachines
- PMID: 33378156
- PMCID: PMC7995253
- DOI: 10.1021/acsami.0c18221
3D-Printed Multi-Stimuli-Responsive Mobile Micromachines
Abstract
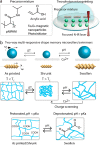
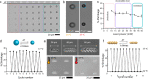
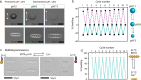
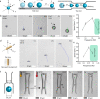
Magnetically actuated and controlled mobile micromachines have the potential to be a key enabler for various wireless lab-on-a-chip manipulations and minimally invasive targeted therapies. However, their embodied, or physical, task execution capabilities that rely on magnetic programming and control alone can curtail their projected performance and functional diversity. Integration of stimuli-responsive materials with mobile magnetic micromachines can enhance their design toolbox, enabling independently controlled new functional capabilities to be defined. To this end, here, we show three-dimensional (3D) printed size-controllable hydrogel magnetic microscrews and microrollers that respond to changes in magnetic fields, temperature, pH, and divalent cations. We show two-way size-controllable microscrews that can reversibly swell and shrink with temperature, pH, and divalent cations for multiple cycles. We present the spatial adaptation of these microrollers for penetration through narrow channels and their potential for controlled occlusion of small capillaries (30 μm diameter). We further demonstrate one-way size-controllable microscrews that can swell with temperature up to 65% of their initial length. These hydrogel microscrews, once swollen, however, can only be degraded enzymatically for removal. Our results can inspire future applications of 3D- and 4D-printed multifunctional mobile microrobots for precisely targeted obstructive interventions (e.g., embolization) and lab- and organ-on-a-chip manipulations.
Keywords: 3D printing; 4D printing; micromachine; microrobot; stimuli-responsive materials.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

References
-
- Qiu F. M.; Fujita S.; Mhanna R.; Zhang L.; Simona B. R.; Nelson B. J. Magnetic Helical Microswimmers Functionalized with Lipoplexes for Targeted Gene Delivery. Adv. Funct. Mater. 2015, 25 (11), 1666–1671. 10.1002/adfm.201403891. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

