Characterization of ecotin homologs from Campylobacter rectus and Campylobacter showae
- PMID: 33378351
- PMCID: PMC7773321
- DOI: 10.1371/journal.pone.0244031
Characterization of ecotin homologs from Campylobacter rectus and Campylobacter showae
Abstract
Ecotin, first described in Escherichia coli, is a potent inhibitor of a broad range of serine proteases including those typically released by the innate immune system such as neutrophil elastase (NE). Here we describe the identification of ecotin orthologs in various Campylobacter species, including Campylobacter rectus and Campylobacter showae residing in the oral cavity and implicated in the development and progression of periodontal disease in humans. To investigate the function of these ecotins in vitro, the orthologs from C. rectus and C. showae were recombinantly expressed and purified from E. coli. Using CmeA degradation/protection assays, fluorescence resonance energy transfer and NE activity assays, we found that ecotins from C. rectus and C. showae inhibit NE, factor Xa and trypsin, but not the Campylobacter jejuni serine protease HtrA or its ortholog in E. coli, DegP. To further evaluate ecotin function in vivo, an E. coli ecotin-deficient mutant was complemented with the C. rectus and C. showae homologs. Using a neutrophil killing assay, we demonstrate that the low survival rate of the E. coli ecotin-deficient mutant can be rescued upon expression of ecotins from C. rectus and C. showae. In addition, the C. rectus and C. showae ecotins partially compensate for loss of N-glycosylation and increased protease susceptibility in the related pathogen, Campylobacter jejuni, thus implicating a similar role for these proteins in the native host to cope with the protease-rich environment of the oral cavity.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
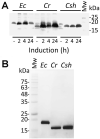
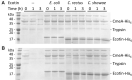

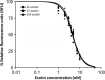

References
-
- Shapiro SD. Proteinases in chronic obstructive pulmonary disease. Biochem Soc Trans. 2002;30(2):98–102. . - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

