NeuroPAL: A Multicolor Atlas for Whole-Brain Neuronal Identification in C. elegans
- PMID: 33378642
- PMCID: PMC10494711
- DOI: 10.1016/j.cell.2020.12.012
NeuroPAL: A Multicolor Atlas for Whole-Brain Neuronal Identification in C. elegans
Abstract
Comprehensively resolving neuronal identities in whole-brain images is a major challenge. We achieve this in C. elegans by engineering a multicolor transgene called NeuroPAL (a neuronal polychromatic atlas of landmarks). NeuroPAL worms share a stereotypical multicolor fluorescence map for the entire hermaphrodite nervous system that resolves all neuronal identities. Neurons labeled with NeuroPAL do not exhibit fluorescence in the green, cyan, or yellow emission channels, allowing the transgene to be used with numerous reporters of gene expression or neuronal dynamics. We showcase three applications that leverage NeuroPAL for nervous-system-wide neuronal identification. First, we determine the brainwide expression patterns of all metabotropic receptors for acetylcholine, GABA, and glutamate, completing a map of this communication network. Second, we uncover changes in cell fate caused by transcription factor mutations. Third, we record brainwide activity in response to attractive and repulsive chemosensory cues, characterizing multimodal coding for these stimuli.
Keywords: C. elegan; atlas; expression pattern; nervous system; whole nervous sytem imaging.
Copyright © 2020 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Interests The authors declare no competing interests.
Figures

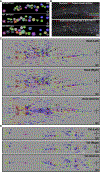




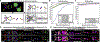
References
-
- Ahrens MB, Orger MB, Robson DN, Li JM, and Keller PJ (2013). Whole-brain functional imaging at cellular resolution using light-sheet microscopy. Nat. Methods 10, 413–420. - PubMed
-
- Bargmann CI, and Horvitz HR (1991). Chemosensory neurons with overlapping functions direct chemotaxis to multiple chemicals in C. elegans. Neuron 7, 729–742. - PubMed
-
- Bargmann CI, and Marder E (2013). From the connectome to brain function. Nat. Methods 10, 483–490. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

