Diabetic fibrosis
- PMID: 33378699
- PMCID: PMC7867637
- DOI: 10.1016/j.bbadis.2020.166044
Diabetic fibrosis
Abstract
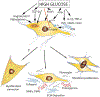
Diabetes-associated morbidity and mortality is predominantly due to complications of the disease that may cause debilitating conditions, such as heart and renal failure, hepatic insufficiency, retinopathy or peripheral neuropathy. Fibrosis, the excessive and inappropriate deposition of extracellular matrix in various tissues, is commonly found in patients with advanced type 1 or type 2 diabetes, and may contribute to organ dysfunction. Hyperglycemia, lipotoxic injury and insulin resistance activate a fibrotic response, not only through direct stimulation of matrix synthesis by fibroblasts, but also by promoting a fibrogenic phenotype in immune and vascular cells, and possibly also by triggering epithelial and endothelial cell conversion to a fibroblast-like phenotype. High glucose stimulates several fibrogenic pathways, triggering reactive oxygen species generation, stimulating neurohumoral responses, activating growth factor cascades (such as TGF-β/Smad3 and PDGFs), inducing pro-inflammatory cytokines and chemokines, generating advanced glycation end-products (AGEs) and stimulating the AGE-RAGE axis, and upregulating fibrogenic matricellular proteins. Although diabetes-activated fibrogenic signaling has common characteristics in various tissues, some organs, such as the heart, kidney and liver develop more pronounced and clinically significant fibrosis. This review manuscript summarizes current knowledge on the cellular and molecular pathways involved in diabetic fibrosis, discussing the fundamental links between metabolic perturbations and fibrogenic activation, the basis for organ-specific differences, and the promises and challenges of anti-fibrotic therapies for diabetic patients.
Keywords: Diabetes; Extracellular matrix; Fibroblast; Fibrosis; Growth factors; Matricellular proteins.
Copyright © 2020 Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

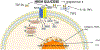
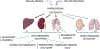
References
-
- Zimmet P, Alberti KG, Magliano DJ, Bennett PH, Diabetes mellitus statistics on prevalence and mortality: facts and fallacies, Nat Rev Endocrinol, 12 (2016) 616–622. - PubMed
-
- Gregg EW, Sattar N, Ali MK, The changing face of diabetes complications, Lancet Diabetes Endocrinol, 4 (2016) 537–547. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

