Comparison of Four SARS-CoV-2 Neutralization Assays
- PMID: 33379160
- PMCID: PMC7824240
- DOI: 10.3390/vaccines9010013
Comparison of Four SARS-CoV-2 Neutralization Assays
Abstract
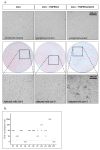
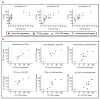
Neutralizing antibodies are a major correlate of protection for many viruses including the novel coronavirus SARS-CoV-2. Thus, vaccine candidates should potently induce neutralizing antibodies to render effective protection from infection. A variety of in vitro assays for the detection of SARS-CoV-2 neutralizing antibodies has been described. However, validation of the different assays against each other is important to allow comparison of different studies. Here, we compared four different SARS-CoV-2 neutralization assays using the same set of patient samples. Two assays used replication competent SARS-CoV-2, a focus forming assay and a TCID50-based assay, while the other two assays used replication defective lentiviral or vesicular stomatitis virus (VSV)-based particles pseudotyped with SARS-CoV-2 spike. All assays were robust and produced highly reproducible neutralization titers. Titers of neutralizing antibodies correlated well between the different assays and with the titers of SARS-CoV-2 S-protein binding antibodies detected in an ELISA. Our study showed that commonly used SARS-CoV-2 neutralization assays are robust and that results obtained with different assays are comparable.
Keywords: SARS-CoV-2; neutralization assay; neutralizing antibodies; pseudotype virus.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- World Health Organization WHO Coronavirus Disease (COVID-19) Dashboard. [(accessed on 22 December 2020)]; Available online: https://covid19.who.int.
-
- FIND SARS-CoV-2 Diagnostic Pipeline. [(accessed on 22 December 2020)]; Available online: https://www.finddx.org/covid-19/pipeline/
-
- Meyer B., Torriani G., Yerly S., Mazza L., Calame A., Arm-Vernez I., Zimmer G., Agoritsas T., Stirnemann J., Spechbach H., et al. Validation of a commercially available SARS-CoV-2 serological immunoassay. Clin. Microbiol. Infect. 2020;26:1386–1394. doi: 10.1016/j.cmi.2020.06.024. - DOI - PMC - PubMed
-
- Grzelak L., Temmam S., Planchais C., Demeret C., Tondeur L., Huon C., Guivel-Benhassine F., Staropoli I., Chazal M., Dufloo J., et al. A comparison of four serological assays for detecting anti-SARS-CoV-2 antibodies in human serum samples from different populations. Sci. Transl. Med. 2020;12:eabc3103. doi: 10.1126/scitranslmed.abc3103. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

