A Potential Atypical Case of Rabbit Haemorrhagic Disease in a Dwarf Rabbit
- PMID: 33379183
- PMCID: PMC7823764
- DOI: 10.3390/ani11010040
A Potential Atypical Case of Rabbit Haemorrhagic Disease in a Dwarf Rabbit
Abstract
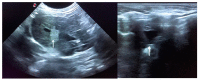
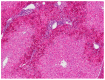
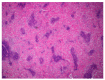
Rabbit haemorrhagic disease (RHD) is a highly contagious infectious disease of European wild and domestic rabbits. Rabbit haemorrhagic disease virus (RHDV, GI.1) emerged in 1986 in Europe, rapidly spreading all over the world. Several genotypes of RHDV have been recognised over time, but in 2010, a new virus (RHDV2/RHDVb, GI.2) emerged and progressively replaced the previous RHDV strains, due to the lack of cross-immunity conferred between RHDV and RHDV2. RHDV2 has a high mutation rate, similarly to the other calivirus and recombines with strains of RHDV and non-pathogenic calicivirus (GI.4), ensuring the continuous emergence of new field strains. Although this poses a threat to the already endangered European rabbit species, the available vaccines against RHDV2 and the compliance of biosafety measures seem to be controlling the infection in the rabbit industry Pet rabbits, especially when kept indoor, are considered at lower risk of infections, although RHDV2 and myxoma virus (MYXV) constitute a permanent threat due to transmission via insects. Vaccination against these viruses is therefore recommended every 6 months (myxomatosis) or annually (rabbit haemorrhagic disease). The combined immunization for myxomatosis and RHDV through a commercially available bivalent vaccine with RHDV antigen has been extensively used (Nobivac® Myxo-RHD, MSD, Kenilworth, NJ, USA). This vaccine however does not confer proper protection against the RHDV2, thus the need for a rabbit clinical vaccination protocol update. Here we report a clinical case of hepatitis and alteration of coagulation in a pet rabbit that had been vaccinated with the commercially available bivalent vaccine against RHDV and tested positive to RHDV2 after death. The animal developed a prolonged and atypical disease, compatible with RHD. The virus was identified to be an RHDV2 recombinant strain, with the structural backbone of RHDV2 (GI.2) and the non-structural genes of non-pathogenic-A1 strains (RCV-A1, GI.4). Although confirmation of the etiological agent was only made after death, the clinical signs and analytic data were very suggestive of RHD.
Keywords: European rabbit; Oryctolagus cuniculus; atypical clinical course; pet rabbit; rabbit haemorrhagic disease; subacute.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Lees A.C., Bell D.J. A conservation paradox for the 21st century: The European wild rabbit Oryctolagus cuniculus, an invasive alien and an endangered native species. Mamm. Rev. 2008;38:304–320. doi: 10.1111/j.1365-2907.2008.00116.x. - DOI
-
- Neimanis A., Larsson Pettersson U., Huang N., Gavier-Widén D., Strive T. Elucidation of the pathology and tissue distribution of Lagovirus europaeus GI.2/RHDV2 (rabbit haemorrhagic disease virus 2) in young and adult rabbits (Oryctolagus cuniculus) Vet. Res. 2018;49:46. doi: 10.1186/s13567-018-0540-z. - DOI - PMC - PubMed
-
- OIE, 2019: Rabbit Haemorrhagic Disease (Technical Disease Card). 1–7. [(accessed on 3 October 2020)]; Available online: https://www.oie.int/fileadmin/Home/eng/Animal_Health_in_the_World/docs/p....
-
- Ramiro-Ibáñez F., Martín-Alonso J.M., García Palencia P., Parra F., Alonso C. Macrophage tropism of rabbit hemorrhagic disease virus is associated with vascular pathology. Virus Res. 1999;60:21–28. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

