Connexins and cAMP Cross-Talk in Cancer Progression and Metastasis
- PMID: 33379194
- PMCID: PMC7795795
- DOI: 10.3390/cancers13010058
Connexins and cAMP Cross-Talk in Cancer Progression and Metastasis
Abstract
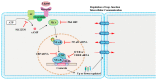
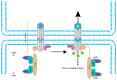
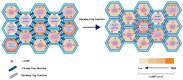
Connexin-containing gap junctions mediate the direct exchange of small molecules between cells, thus promoting cell-cell communication. Connexins (Cxs) have been widely studied as key tumor-suppressors. However, certain Cx subtypes, such as Cx43 and Cx26, are overexpressed in metastatic tumor lesions. Cyclic adenosine monophosphate (cAMP) signaling regulates Cx expression and function via transcriptional control and phosphorylation. cAMP also passes through gap junction channels between adjacent cells, regulating cell cycle progression, particularly in cancer cell populations. Low levels of cAMP are sufficient to activate key effectors. The present review evaluates the mechanisms underlying Cx regulation by cAMP signaling and the role of gap junctions in cancer progression and metastasis. A deeper understanding of these processes might facilitate the development of novel anticancer drugs.
Keywords: cAMP; cancer; connexin; metastasis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

