Possible Adverse Effects of Food Additive E171 (Titanium Dioxide) Related to Particle Specific Human Toxicity, Including the Immune System
- PMID: 33379217
- PMCID: PMC7795714
- DOI: 10.3390/ijms22010207
Possible Adverse Effects of Food Additive E171 (Titanium Dioxide) Related to Particle Specific Human Toxicity, Including the Immune System
Abstract
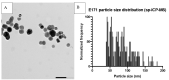
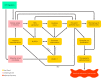
Titanium dioxide (TiO2) is used as a food additive (E171) and can be found in sauces, icings, and chewing gums, as well as in personal care products such as toothpaste and pharmaceutical tablets. Along with the ubiquitous presence of TiO2 and recent insights into its potentially hazardous properties, there are concerns about its application in commercially available products. Especially the nano-sized particle fraction (<100 nm) of TiO2 warrants a more detailed evaluation of potential adverse health effects after ingestion. A workshop organized by the Dutch Office for Risk Assessment and Research (BuRO) identified uncertainties and knowledge gaps regarding the gastrointestinal absorption of TiO2, its distribution, the potential for accumulation, and induction of adverse health effects such as inflammation, DNA damage, and tumor promotion. This review aims to identify and evaluate recent toxicological studies on food-grade TiO2 and nano-sized TiO2 in ex-vivo, in-vitro, and in-vivo experiments along the gastrointestinal route, and to postulate an Adverse Outcome Pathway (AOP) following ingestion. Additionally, this review summarizes recommendations and outcomes of the expert meeting held by the BuRO in 2018, in order to contribute to the hazard identification and risk assessment process of ingested TiO2.
Keywords: E171; TiO2; adverse health effects; food additive; food safety; mode of action; nano size; nanomaterial; oral exposure; review; titanium dioxide; toxicity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

