Kaempferol Inhibits Zearalenone-Induced Oxidative Stress and Apoptosis via the PI3K/Akt-Mediated Nrf2 Signaling Pathway: In Vitro and In Vivo Studies
- PMID: 33379332
- PMCID: PMC7794799
- DOI: 10.3390/ijms22010217
Kaempferol Inhibits Zearalenone-Induced Oxidative Stress and Apoptosis via the PI3K/Akt-Mediated Nrf2 Signaling Pathway: In Vitro and In Vivo Studies
Abstract
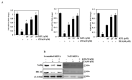
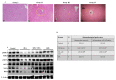
In this study, kaempferol (KFL) shows hepatoprotective activity against zearalenone (ZEA)-induced oxidative stress and its underlying mechanisms in in vitro and in vivo models were investigated. Oxidative stress plays a critical role in the pathophysiology of various hepatic ailments and is normally regulated by reactive oxygen species (ROS). ZEA is a mycotoxin known to exert toxicity via inflammation and ROS accumulation. This study aims to explore the protective role of KFL against ZEA-triggered hepatic injury via the PI3K/Akt-regulated Nrf2 pathway. KFL augmented the phosphorylation of PI3K and Akt, which may stimulate antioxidative and antiapoptotic signaling in hepatic cells. KFL upregulated Nrf2 phosphorylation and the expression of antioxidant genes HO-1 and NQO-1 in a dose-dependent manner under ZEA-induced oxidative stress. Nrf2 knockdown via small-interfering RNA (siRNA) inhibited the KFL-mediated defence against ZEA-induced hepatotoxicity. In vivo studies showed that KFL decreased inflammation and lipid peroxidation and increased H2O2 scavenging and biochemical marker enzyme expression. KFL was able to normalize the expression of liver antioxidant enzymes SOD, CAT and GSH and showed a protective effect against ZEA-induced pathophysiology in the livers of mice. These outcomes demonstrate that KFL possesses notable hepatoprotective roles against ZEA-induced damage in vivo and in vitro. These protective properties of KFL may occur through the stimulation of Nrf2/HO-1 cascades and PI3K/Akt signaling.
Keywords: Nrf2; PI3K/Akt; apoptosis; hepatotoxicity; kaempferol; zearalenone.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- Rajput S.A., Sun L., Zhang N.-Y., Khalil M.M., Ling Z., Chong L., Wang S., Rajput I.R., Bloch D.M., Khan F.A. Grape seed proanthocyanidin extract alleviates aflatoxinB1-induced immunotoxicity and oxidative stress via modulation of NF-κB and Nrf2 signaling pathways in broilers. Toxins. 2019;11:23. doi: 10.3390/toxins11010023. - DOI - PMC - PubMed
-
- Fernández-Cruz M.L., Mansilla M.L., Tadeo J.L. Mycotoxins in fruits and their processed products: Analysis, occurrence and health implications. J. Adv. Res. 2010;1:113–122. doi: 10.1016/j.jare.2010.03.002. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

