Oxygen Transport during Ex Situ Machine Perfusion of Donor Livers Using Red Blood Cells or Artificial Oxygen Carriers
- PMID: 33379394
- PMCID: PMC7795786
- DOI: 10.3390/ijms22010235
Oxygen Transport during Ex Situ Machine Perfusion of Donor Livers Using Red Blood Cells or Artificial Oxygen Carriers
Abstract
Oxygenated ex situ machine perfusion of donor livers is an alternative for static cold preservation that can be performed at temperatures from 0 °C to 37 °C. Organ metabolism depends on oxygen to produce adenosine triphosphate and temperatures below 37 °C reduce the metabolic rate and oxygen requirements. The transport and delivery of oxygen in machine perfusion are key determinants in preserving organ viability and cellular function. Oxygen delivery is more challenging than carbon dioxide removal, and oxygenation of the perfusion fluid is temperature dependent. The maximal oxygen content of water-based solutions is inversely related to the temperature, while cellular oxygen demand correlates positively with temperature. Machine perfusion above 20 °C will therefore require an oxygen carrier to enable sufficient oxygen delivery to the liver. Human red blood cells are the most physiological oxygen carriers. Alternative artificial oxygen transporters are hemoglobin-based oxygen carriers, perfluorocarbons, and an extracellular oxygen carrier derived from a marine invertebrate. We describe the principles of oxygen transport, delivery, and consumption in machine perfusion for donor livers using different oxygen carrier-based perfusion solutions and we discuss the properties, advantages, and disadvantages of these carriers and their use.
Keywords: artificial oxygen carriers; carbon dioxide; gas transport; liver; machine perfusion; oxygen; temperature.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
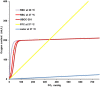
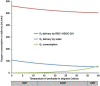

References
-
- Adam R., Karam V., Cailliez V., O Grady J.G., Mirza D., Cherqui D., Klempnauer J., Salizzoni M., Pratschke J., Jamieson N., et al. 2018 Annual Report of the European Liver Transplant Registry (ELTR)-50-year evolution of liver transplantation. Transpl. Int. 2018;31:1293–1317. doi: 10.1111/tri.13358. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

