Palmitoylation of acetylated tubulin and association with ceramide-rich platforms is critical for ciliogenesis
- PMID: 33380429
- PMCID: PMC7903138
- DOI: 10.1194/jlr.RA120001190
Palmitoylation of acetylated tubulin and association with ceramide-rich platforms is critical for ciliogenesis
Abstract
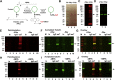
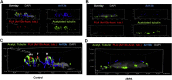
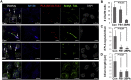
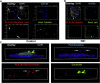
Microtubules are polymers composed of αβ-tubulin subunits that provide structure to cells and play a crucial role in in the development and function of neuronal processes and cilia, microtubule-driven extensions of the plasma membrane that have sensory (primary cilia) or motor (motile cilia) functions. To stabilize microtubules in neuronal processes and cilia, α tubulin is modified by the posttranslational addition of an acetyl group, or acetylation. We discovered that acetylated tubulin in microtubules interacts with the membrane sphingolipid, ceramide. However, the molecular mechanism and function of this interaction are not understood. Here, we show that in human induced pluripotent stem cell-derived neurons, ceramide stabilizes microtubules, which indicates a similar function in cilia. Using proximity ligation assays, we detected complex formation of ceramide with acetylated tubulin in Chlamydomonas reinhardtii flagella and cilia of human embryonic kidney (HEK293T) cells, primary cultured mouse astrocytes, and ependymal cells. Using incorporation of palmitic azide and click chemistry-mediated addition of fluorophores, we show that a portion of acetylated tubulin is S-palmitoylated. S-palmitoylated acetylated tubulin is colocalized with ceramide-rich platforms in the ciliary membrane, and it is coimmunoprecipitated with Arl13b, a GTPase that mediates transport of proteins into cilia. Inhibition of S-palmitoylation with 2-bromo palmitic acid or inhibition of ceramide biosynthesis with fumonisin B1 reduces formation of the Arl13b-acetylated tubulin complex and its transport into cilia, concurrent with impairment of ciliogenesis. Together, these data show, for the first time, that ceramide-rich platforms mediate membrane anchoring and interaction of S-palmitoylated proteins that are critical for cilium formation, stabilization, and function.
Keywords: Arl13b; acetylation; cell biology; ceramide; cilia; lipid rafts; lipids; microtubules; palmitoylation; tubulin.
Published by Elsevier Inc.
Conflict of interest statement
Conflict of interest The authors declare that they have no conflicts of interest with the contents of this article.
Figures








References
-
- Jiang X., Zhu Z., Qin H., Tripathi P., Zhong L., Elsherbini A., Karki S., Crivelli S.M., Zhi W., Wang G., Spassieva S.D., Bieberich E. Visualization of ceramide-associated proteins in ceramide-rich platforms using a cross-linkable ceramide analog and proximity ligation assays with anti-ceramide antibody. Front. Cell Dev. Biol. 2019;7:166. - PMC - PubMed
-
- Cho W., Stahelin R.V. Membrane-protein interactions in cell signaling and membrane trafficking. Annu. Rev. Biophys. Biomol. Struct. 2005;34:119–151. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

