Microglia Elimination Increases Neural Circuit Connectivity and Activity in Adult Mouse Cortex
- PMID: 33380470
- PMCID: PMC7888230
- DOI: 10.1523/JNEUROSCI.2140-20.2020
Microglia Elimination Increases Neural Circuit Connectivity and Activity in Adult Mouse Cortex
Abstract
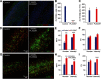
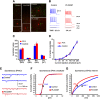
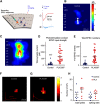
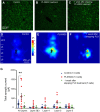
Microglia have crucial roles in sculpting synapses and maintaining neural circuits during development. To test the hypothesis that microglia continue to regulate neural circuit connectivity in adult brain, we have investigated the effects of chronic microglial depletion, via CSF1R inhibition, on synaptic connectivity in the visual cortex in adult mice of both sexes. We find that the absence of microglia dramatically increases both excitatory and inhibitory synaptic connections to excitatory cortical neurons assessed with functional circuit mapping experiments in acutely prepared adult brain slices. Microglia depletion leads to increased densities and intensities of perineuronal nets. Furthermore, in vivo calcium imaging across large populations of visual cortical neurons reveals enhanced neural activities of both excitatory neurons and parvalbumin-expressing interneurons in the visual cortex following microglia depletion. These changes recover following adult microglia repopulation. In summary, our new results demonstrate a prominent role of microglia in sculpting neuronal circuit connectivity and regulating subsequent functional activity in adult cortex.SIGNIFICANCE STATEMENT Microglia are the primary immune cell of the brain, but recent evidence supports that microglia play an important role in synaptic sculpting during development. However, it remains unknown whether and how microglia regulate synaptic connectivity in adult brain. Our present work shows chronic microglia depletion in adult visual cortex induces robust increases in perineuronal nets, and enhances local excitatory and inhibitory circuit connectivity to excitatory neurons. Microglia depletion increases in vivo neural activities of both excitatory neurons and parvalbumin inhibitory neurons. Our new results reveal new potential avenues to modulate adult neural plasticity by microglia manipulation to better treat brain disorders, such as Alzheimer's disease.
Keywords: excitatory; parvalbumin; perineuronal nets; plasticity; synaptic connections; visual cortex.
Copyright © 2021 the authors.
Figures


Comment in
-
Commentary: How Do Microglia Regulate Neural Circuit Connectivity and Activity in the Adult Brain?Neurosci Insights. 2022 Jan 24;17:26331055211071124. doi: 10.1177/26331055211071124. eCollection 2022. Neurosci Insights. 2022. PMID: 35098130 Free PMC article.
Similar articles
-
Commentary: How Do Microglia Regulate Neural Circuit Connectivity and Activity in the Adult Brain?Neurosci Insights. 2022 Jan 24;17:26331055211071124. doi: 10.1177/26331055211071124. eCollection 2022. Neurosci Insights. 2022. PMID: 35098130 Free PMC article.
-
Partial microglial depletion through inhibition of colony-stimulating factor 1 receptor improves synaptic plasticity and cognitive performance in aged mice.Exp Neurol. 2025 May;387:115186. doi: 10.1016/j.expneurol.2025.115186. Epub 2025 Feb 14. Exp Neurol. 2025. PMID: 39956381
-
p75 Neurotrophin Receptor Activation Regulates the Timing of the Maturation of Cortical Parvalbumin Interneuron Connectivity and Promotes Juvenile-like Plasticity in Adult Visual Cortex.J Neurosci. 2019 Jun 5;39(23):4489-4510. doi: 10.1523/JNEUROSCI.2881-18.2019. Epub 2019 Apr 1. J Neurosci. 2019. PMID: 30936240 Free PMC article.
-
Ferret Interneurons Defy Expectations.Neuron. 2017 Mar 8;93(5):985-987. doi: 10.1016/j.neuron.2017.02.037. Neuron. 2017. PMID: 28279359 Review.
-
Microglial Depletion, a New Tool in Neuroinflammatory Disorders: Comparison of Pharmacological Inhibitors of the CSF-1R.Glia. 2025 Apr;73(4):686-700. doi: 10.1002/glia.24664. Epub 2024 Dec 24. Glia. 2025. PMID: 39719687 Free PMC article. Review.
Cited by
-
Microglial Modulation of Synaptic Maturation, Activity, and Plasticity.Adv Neurobiol. 2024;37:209-219. doi: 10.1007/978-3-031-55529-9_12. Adv Neurobiol. 2024. PMID: 39207694 Review.
-
Glia Regulate the Development, Function, and Plasticity of the Visual System From Retina to Cortex.Front Neural Circuits. 2022 Feb 1;16:826664. doi: 10.3389/fncir.2022.826664. eCollection 2022. Front Neural Circuits. 2022. PMID: 35177968 Free PMC article. Review.
-
Fractalkine/CX3CR1-Dependent Modulation of Synaptic and Network Plasticity in Health and Disease.Neural Plast. 2023 Jan 4;2023:4637073. doi: 10.1155/2023/4637073. eCollection 2023. Neural Plast. 2023. PMID: 36644710 Free PMC article. Review.
-
The "Loss" of Perineuronal Nets in Alzheimer's Disease: Missing or Hiding in Plain Sight?Front Integr Neurosci. 2022 May 25;16:896400. doi: 10.3389/fnint.2022.896400. eCollection 2022. Front Integr Neurosci. 2022. PMID: 35694184 Free PMC article.
-
Embryonic Pericytes Promote Microglial Homeostasis and Their Effects on Neural Progenitors in the Developing Cerebral Cortex.J Neurosci. 2022 Jan 19;42(3):362-376. doi: 10.1523/JNEUROSCI.1201-21.2021. Epub 2021 Nov 24. J Neurosci. 2022. PMID: 34819341 Free PMC article.
References
-
- Brückner G, Grosche J, Schmidt S, Hartig W, Margolis RU, Delpech B, Seidenbecher CI, Czaniera R, Schachner M (2000) Postnatal development of perineuronal nets in wild-type mice and in a mutant deficient in tenascin-R. J Comp Neurol 428:616–629. 10.1002/1096-9861(20001225)428:4<616::AID-CNE3>3.0.CO;2-K - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
