Metagenomic Next-Generation Sequencing of Cerebrospinal Fluid for the Diagnosis of External Ventricular and Lumbar Drainage-Associated Ventriculitis and Meningitis
- PMID: 33381092
- PMCID: PMC7767851
- DOI: 10.3389/fmicb.2020.596175
Metagenomic Next-Generation Sequencing of Cerebrospinal Fluid for the Diagnosis of External Ventricular and Lumbar Drainage-Associated Ventriculitis and Meningitis
Abstract
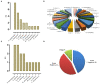
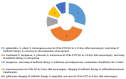
Metagenomic next-generation sequencing (mNGS) has become a widely used technology that can accurately detect individual pathogens. This prospective study was performed between February 2019 and September 2019 in one of the largest clinical neurosurgery centers in China. The study aimed to evaluate the performance of mNGS on cerebrospinal fluid (CSF) from neurosurgical patients for the diagnosis of external ventricular and lumbar drainage (EVD/LD)-associated ventriculitis and meningitis (VM). We collected CSF specimens from neurosurgical patients with EVD/LD for more than 24 h to perform conventional microbiological studies and mNGS analyses in a pairwise manner. We also investigated the usefulness of mNGS of CSF for the diagnosis of EVD/LD-associated VM. In total, 102 patients were enrolled in this study and divided into three groups, including confirmed VM (cVM) (39), suspected VM (sVM) (49), and non-VM (nVM) (14) groups. Of all the patients, mNGS detected 21 Gram-positive bacteria, 20 Gram-negative bacteria, and five fungi. The three primary bacteria detected were Staphylococcus epidermidis (9), Acinetobacter baumannii (5), and Staphylococcus aureus (3). The mNGS-positive coincidence rate of confirmed EVD/LD-associated VM was 61.54% (24/39), and the negative coincidence rate of the nVM group was 100% (14/14). Of 15 VM pathogens not identified by mNGS in the cVM group, eight were negative with mNGS and seven were inconsistent with the conventional microbiological identification results. In addition, mNGS identified pathogens in 22 cases that were negative using conventional methods; of them, 10 patients received a favorable clinical treatment; thus, showing the benefit of mNGS-guided therapy.
Keywords: EVD; LD; diagnosis; metagenomic next-generation sequencing (mNGS); ventriculitis and meningitis.
Copyright © 2020 Qian, Shi, Li, Wang, Ma, Zhang, Shao, Zheng and Zhang.
Conflict of interest statement
YWS was employed by Nanjing Geneseeq Technology Inc. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Brain Trauma Foundation American Association of Neurological Surgeons Congress of Neurological Surgeons Joint Section on Neurotrauma and Critical Care AANS/CN. Bratton S. L., Chestnut R. M., et al. (2007). Guidelines for the management of severe traumatic brain injury. IV. infection prophylaxis. J. Neurotrauma 24 (Suppl. 1), S26–31. 10.1089/neu.2007.999 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

