The Structure, Function, and Mechanisms of Action of Enterovirus Non-structural Protein 2C
- PMID: 33381104
- PMCID: PMC7767853
- DOI: 10.3389/fmicb.2020.615965
The Structure, Function, and Mechanisms of Action of Enterovirus Non-structural Protein 2C
Abstract
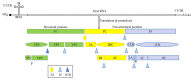
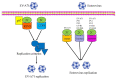
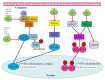
Enteroviruses are a group of RNA viruses belonging to the family Picornaviridae. They include human enterovirus groups A, B, C, and D as well as non-human enteroviruses. Enterovirus infections can lead to hand, foot, and mouth disease and herpangina, whose clinical manifestations are often mild, although some strains can result in severe neurological complications such as encephalitis, myocarditis, meningitis, and poliomyelitis. To date, research on enterovirus non-structural proteins has mainly focused on the 2A and 3C proteases and 3D polymerase. However, another non-structural protein, 2C, is the most highly conserved protein, and plays a vital role in the enterovirus life cycle. There are relatively few studies on this protein. Previous studies have demonstrated that enterovirus 2C is involved in virus uncoating, host cell membrane rearrangements, RNA replication, encapsidation, morphogenesis, ATPase, helicase, and chaperoning activities. Despite ongoing research, little is known about the pathogenesis of enterovirus 2C proteins in viral replication or in the host innate immune system. In this review, we discuss and summarize the current understanding of the structure, function, and mechanism of the enterovirus 2C proteins, focusing on the key mutations and motifs involved in viral infection, replication, and immune regulation. We also focus on recent progress in research into the role of 2C proteins in regulating the pattern recognition receptors and type I interferon signaling pathway to facilitate viral replication. Given these functions and mechanisms, the potential application of the 2C proteins as a target for anti-viral drug development is also discussed. Future studies will focus on the determination of more crystal structures of enterovirus 2C proteins, which might provide more potential targets for anti-viral drug development against enterovirus infections.
Keywords: 2C protein; anti-viral drug; enterovirus; function; host immune response; structure; type I IFNs.
Copyright © 2020 Wang, Wang, Zhao, Hua and Du.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

