Resolving the Paradox of Colon Cancer Through the Integration of Genetics, Immunology, and the Microbiota
- PMID: 33381121
- PMCID: PMC7768083
- DOI: 10.3389/fimmu.2020.600886
Resolving the Paradox of Colon Cancer Through the Integration of Genetics, Immunology, and the Microbiota
Abstract
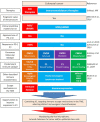
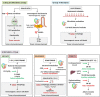
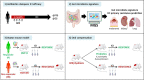
While colorectal cancers (CRC) are paradigmatic tumors invaded by effector memory lymphocytes, the mechanisms accounting for the relative resistance of MSI negative CRC to immunogenic cell death mediated by oxaliplatin and immune checkpoint inhibitors has remained an open conundrum. Here, we propose the viewpoint where its microenvironmental contexture could be explained -at least in part- by macroenvironmental cues constituted by the complex interplay between the epithelial barrier, its microbial ecosystem, and the local immune system. Taken together this dynamic ménage-à-trois offers novel coordinated actors of the humoral and cellular immune responses actionable to restore sensitivity to immune checkpoint inhibition. Solving this paradox involves breaking tolerance to crypt stem cells by inducing the immunogenic apoptosis of ileal cells in the context of an ileal microbiome shifted towards immunogenic bacteria using cytotoxicants. This manoeuver results in the elicitation of a productive Tfh and B cell dialogue in mesenteric lymph nodes culminating in tumor-specific memory CD8+ T cell responses sparing the normal epithelium.
Keywords: Bacteroides fragilis; Fusobacterium nucleatum; colon cancer; ileum; immune checkpoint; immunity; microbiome.
Copyright © 2020 Fidelle, Yonekura, Picard, Cogdill, Hollebecque, Roberti and Zitvogel.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
-
- Murphy N, Ward HA, Jenab M, Rothwell JA, Boutron-Ruault M-C, Carbonnel F, et al. Heterogeneity of Colorectal Cancer Risk Factors by Anatomical Subsite in 10 European Countries: A Multinational Cohort Study. Clin Gastroenterol Hepatol (2019) 17:1323–31.e6. 10.1016/j.cgh.2018.07.030 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

