Stem Cell Mechanobiology and the Role of Biomaterials in Governing Mechanotransduction and Matrix Production for Tissue Regeneration
- PMID: 33381498
- PMCID: PMC7767888
- DOI: 10.3389/fbioe.2020.597661
Stem Cell Mechanobiology and the Role of Biomaterials in Governing Mechanotransduction and Matrix Production for Tissue Regeneration
Abstract
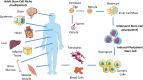
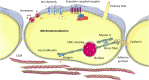
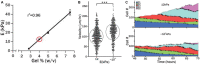
Mechanobiology has underpinned many scientific advances in understanding how biophysical and biomechanical cues regulate cell behavior by identifying mechanosensitive proteins and specific signaling pathways within the cell that govern the production of proteins necessary for cell-based tissue regeneration. It is now evident that biophysical and biomechanical stimuli are as crucial for regulating stem cell behavior as biochemical stimuli. Despite this, the influence of the biophysical and biomechanical environment presented by biomaterials is less widely accounted for in stem cell-based tissue regeneration studies. This Review focuses on key studies in the field of stem cell mechanobiology, which have uncovered how matrix properties of biomaterial substrates and 3D scaffolds regulate stem cell migration, self-renewal, proliferation and differentiation, and activation of specific biological responses. First, we provide a primer of stem cell biology and mechanobiology in isolation. This is followed by a critical review of key experimental and computational studies, which have unveiled critical information regarding the importance of the biophysical and biomechanical cues for stem cell biology. This review aims to provide an informed understanding of the intrinsic role that physical and mechanical stimulation play in regulating stem cell behavior so that researchers may design strategies that recapitulate the critical cues and develop effective regenerative medicine approaches.
Keywords: 2D substrate stiffness; 3D biomaterial stiffness; biomechanical stimuli; biophysical stimuli; computational modeling; regenerative medicine; tissue engineering.
Copyright © 2020 Naqvi and McNamara.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Altmann B., Steinberg T., Giselbrecht S., Gottwald E., Tomakidi P., Bächle-Haas M., et al. (2011). Promotion of osteoblast differentiation in 3D biomaterial micro-chip arrays comprising fibronectin-coated poly(methyl methacrylate) polycarbonate. Biomaterials 32 8947–8956. 10.1016/j.biomaterials.2011.08.023 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

